NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Peterson BS, Trampush J, Maglione M, et al. ADHD Diagnosis and Treatment in Children and Adolescents [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2024 Mar. (Comparative Effectiveness Review, No. 267.)
This section describes studies reporting on a treatment of attention deficit hyperactivity disorder (ADHD). Key points are listed first, followed by a summary of findings section before going into the effects and comparative effects of specific interventions.
5.1. KQ2, ADHD Treatment Key Points
- Head-to-head comparisons between stimulants and non-stimulants did not detect statistically significant differences for most effectiveness outcomes and adverse events.
- We found little evidence that combination therapies of medication plus psychosocial therapies produce better results than medication alone, but existing research evaluated unique combinations of intervention components.
- Despite the large body of research, comparative effectiveness and safety information is limited, and more research is needed to help choose between treatments.
- We did not detect differential treatment effects associated with ADHD presentation, but analyses were based on indirect comparisons and should be interpreted with caution.
- Data were insufficient to assess the effect of co-occurring disorders on treatment effects.
- We found too few studies reporting on diversion to quantify the risk of diversion of pharmacological treatment.
5.2. KQ2, ADHD Treatment Results
We identified 312 studies evaluating a treatment for ADHD.56, 104–110, 113, 114, 116, 118, 122, 123, 125–133, 136–139, 144–151, 154–156, 158, 160, 161, 163–166, 171, 174–176, 178, 180, 193–196, 199–202, 204–209, 212, 215–217, 219–222, 224–229, 232, 235, 236, 238–240, 243, 247–250, 252, 254–259, 261, 262, 264–266, 269–273, 275, 278–281, 286, 288–292, 294–296, 302, 304–306, 308, 310, 313, 317, 318, 320, 321, 324–326, 328–330, 332–335, 337, 341, 343, 345, 348–350, 353, 354, 357, 358, 360, 361, 363, 364, 367, 368, 371–378, 380, 381, 383, 384, 386, 387, 392, 396, 398, 399, 406, 409–411, 414, 418, 419, 425, 426, 428, 430–433, 435, 439–444, 451–461, 466, 471, 472, 474, 476, 478, 480, 481, 483–485, 488–490, 492, 497, 503–505, 507–513, 517, 520–523, 525, 526, 529–535, 538–540, 544, 550–552, 554–557, 560–562, 565, 567–569, 572–575, 577–579, 585, 586, 588–590, 593–598, 601, 602, 604, 606, 608, 610–613, 616–624, 626, 628, 634, 636, 637, 640, 643, 645, 646 Although studies from 1980 were eligible, the earliest treatment studies meeting inclusion criteria were published in 1995. Studies were published in 30 different countries, although about 40 percent were U.S. studies (contributing 127 included studies).
The summary of findings table broadly summarizes the available evidence for the key outcomes across identified treatment studies.
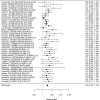
Figure 17 plots the followup periods across treatment studies.
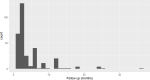
Figure 17
Followup in KQ2 ADHD treatment studies. Notes: ADHD = attention deficit hyperactivity disorder
With few exceptions, studies reported short-term effects.
The potential for risk of bias in Key Question (KQ) 2 studies is documented in Figure 18. The critical appraisal for the individual studies is in Appendix D.
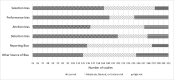
Figure 18
Risk of bias in Key Question 2 ADHD treatment studies. Notes: ADHD = attention deficit hyperactivity disorder
Across studies, selection bias was likely present in multiple identified studies. This was predominantly attributable to highly selected samples and exclusions, or a biased allocation into groups because of study logistics. The review was open to all studies evaluating intervention in youth with a ADHD without further limitations, but some included studies reported a number of additional inclusion and exclusion criteria. Performance bias was noted in half of the included studies. An example of this kind of bias is that participants deviated from protocol medication administration (e.g., parents frequently reduced weekend medication use on their own). Attrition bias was also often noted, with large numbers of participants being unavailable for follow-up assessments. Detection bias was detected in many studies where blinding was not possible or would be very difficult and the outcome assessors (often the parents of the participants) were aware of the participants’ intervention assignment. Reporting bias was also suspected in some of the studies, usually indicating that the study did not report on key ADHD outcomes, and no study protocol was published specifying that prospectively. Other sources of bias were identified in a third of studies, concerning small samples or inadequate descriptions of either the interventions or study flow.
Figure 19 shows the distribution of KQ2 studies with applicability issues. The applicability for the individual studies is documented in Appendix D.

Figure 19
KQ2 ADHD treatment applicability rating. Notes: ADHD = attention deficit hyperactivity disorder, DSM = Diagnostic and Statistical Manual of Mental Disorders, N/A = Not applicable
Applicability issues primarily concerned the participant samples in the identified studies. Some of the samples were less diverse than the typical population seen in clinical practice, often because of very strict inclusion criteria for the study (e.g., excluding children with co-occurring disorders). A large number of studies did not report any characteristics that flagged the comparator or the setting as different from the level of care in the community (listed as not applicable in the figure).
The 78 populations studied were predominately males, and some studies (2%) were restricted to boys; samples included on average a quarter of female participants. The youngest children in individual studies were three years old. Race and ethnicity demographics were not mentioned in over half of the studies. For studies that distinguished between ADHD presentations, the most prevalent type was the combined type.
The following sections summarize the effects of interventions on the key outcomes. This is a very broad analysis; however, it is an important question whether ADHD characteristics can be changed at all with interventions. For each section, a narrative summary is followed by a summary of findings table. Summary tables report on each of the key outcomes. Subgroup results are only added to the summary tables when a direct or indirect analysis suggested empirically different results and more than one study contributed to the effect estimate. Additional information on study-specific primary outcomes are documented in the evidence table in the appendix.
5.2.1. Effects of ADHD Treatment on Behavior
The results for any achieved changes in problem behavior (e.g., conduct problems) across the diverse ADHD interventions evaluating a continuous outcome (and reporting sufficient information to allow effect size calculations) showed a positive effect compared to passive control groups (standardized mean difference [SMD] −0.34; confidence interval [CI] −0.49, −18; 34 studies, n=3507). There was evidence of heterogeneity (I-squared 66%). We tested whether the intervention type was a key source of heterogeneity to explain differences in effects; results indicated that effect estimates for behavior depend on the type of intervention (p 0.04). Analyses suggested publication bias (Begg p 0.01, Egger p<0.0001), indicating that publication bias should be considered for individual analyses. We also estimate in a sensitivity analysis whether the result was mainly driven by high risk-of-bias studies; after removing 13 high risk-of-bias studies, the estimate was similar (SMD −0.32; CI −0.48, −0.17). Across studies, only three studies were identified reporting on categorical outcomes (e.g., assessing whether or not behavior had improved). Results indicated reductions in problematic behavior associated with ADHD treatment (RR [relative risk] 0.46; CI 0.24, 0.87; 3 studies, n=154). In this small set of studies, there was no evidence of heterogeneity or publication bias. None of the studies was classified as high risk.
5.2.2. Effects of ADHD Treatment on Broadband Measures
The results for broadband scales describing a child’s behavior more generally showed positive effects of ADHD interventions (SMD 0.39, CI 0.31, 0.47; 72 studies, n=9027). There was evidence of heterogeneity (I-squared 68%). We tested whether the intervention was the key source of heterogeneity to explain differences in effects, but we did not detect an effect (p 0.29). There was no evidence of publication bias. We removed 25 high risk-of-bias studies in a sensitivity analysis, but the effect estimate remained similar (SMD 0.42, CI 0.33, 0.52). Multiple studies also reported on these global impressions as categorical variables and the effect was similar for the categorical broadband measures, indicating improvement associated with ADHD treatment (RR 0.57; CI 0.48, 0.66; 40 studies, n=6033). There was evidence of heterogeneity (I-squared 81%). We tested whether the intervention was the key source of heterogeneity to explain differences in effects, but we did not detect a systematic effect (p 0.34). There was evidence of publication bias (Begg p 0.01, Egger p<0.001) and an alternative estimate using the trim and fill method showed a somewhat smaller effect (RR 0.64; CI 0.55, 0.75). We also conducted a sensitivity analysis to determine whether results are robust when removing six high risk-of-bias studies; the estimate was very similar to the original results (RR 0.57; CI 0.48, 0.68).
5.2.3. Effects of ADHD Treatment on ADHD Symptoms
A large number of studies reported on standardized symptom assessment tools. Standardized mean difference results across studies using continuous data found a positive effect of interventions successfully reducing ADHD symptom severity (SMD −0.47, CI −0.54, −0.40; 150 studies, n=18746). There was evidence of heterogeneity (I-squared 80%). We tested whether the intervention was the key source of heterogeneity to explain differences in effects and found that the reported effect size was not systematically associated with the type of intervention evaluated (p 0.13). There was some indication of publication bias (Begg p 0.09, Egger, p 0.02), but an alternative effect estimate using the trim and fill method found a very similar estimate SMD ‑0.47; CI −0.55, −0.40). Excluding 49 high-risk-of-bias studies in a sensitivity analysis resulted in a similar estimate (SMD −0.47, CI −0.55, −0.38) and heterogeneity was not reduced. A smaller number of studies reported on a dichotomous outcome for ADHD symptoms (e.g., meeting or not meeting an improvement target). Across studies, we found a positive effect of ADHD interventions (RR 1.51, CI 1.23, 1.84; 26 studies, n=3289). We detected heterogeneity (I-squared 67%), but a moderator analysis did not detect the intervention as a source of heterogeneity (p 0.18). There was evidence of publication bias (Begg p<0.004, Egger p<0.001). A more appropriate estimate of the true effect on symptom reduction may be somewhat smaller (RR 1.31, CI 1.06, 1.60). We also removed four high-risk of bias studies in a sensitivity analysis which showed the treatment effect to be robust (RR 1.58, CI 1.24, 2.00) but heterogeneity was not reduced.
5.2.4. Effects of ADHD Treatment on Functional Impairment
The results for functional impairment measures across the diverse interventions in studies reporting on a continuous outcome found a positive effect of ADHD interventions on functional impairment (SMD 0.37; CI 0.20, 0.54; 31 studies, n=3890). There was evidence of heterogeneity (I-squared 82%). We tested whether the intervention was the key source of heterogeneity to explain differences in effects, but we did not detect a systematic effect (p 0.88). There was no significant publication bias. When removing 11 high-risk of bias studies in a sensitivity analysis, the estimate remained similar (SMD 0.40; CI 0.17, 0.62) and heterogeneity was not reduced. Very few studies reported on functional impairment as a categorical variable, and only one study reported sufficient information to compute effect sizes. The study indicated improvement, but the confidence interval was wide (RR 1.29; CI 1.00, 1.66; 1 study, n=332).
5.2.5. Effects of ADHD Treatment on Acceptability of Treatment
Only one study assessed treatment acceptability formally in a rating scale for all groups and reported sufficient detail to compute effect sizes; the study did not find a statistically significant difference between groups (SMD 0.19; CI −0.12, 0.49; 1 study, n=164). One study reported categorical data to express satisfaction with the treatment; the study favored the intervention (RR 0.47; CI 0.32, 0.68; 1 study, n=198). There were insufficient data for further analyses.
5.2.6. Effects of ADHD Treatment on Academic Performance
The results for academic performance changes reported in sufficient detail across the diverse interventions favored ADHD treatment arms, but we did not detect a statistically significant difference between ADHD treatment and passive control groups on academic performance (SMD −0.29; CI −0.62, 0.03; 12 studies, n=1780). There was evidence of heterogeneity (I-squared 88%). We tested whether the intervention was the key source of heterogeneity to explain differences in effects, but the intervention type did not systematically contribute to the heterogeneity of effects (p 0.10). Publication bias tests did not indicate potential bias. Removing two high risk-of-bias studies in a sensitivity analysis showed a smaller effect, and the difference between groups remained not statistically significant (SMD −0.29; CI −0.69, 0.10). None of the studies comparing to a control group reported on a categorical outcome in sufficient detail to allow effect size calculation.
5.2.7. Effects of ADHD Treatment on Appetite Changes
We identified several studies that reported on a continuous measure to capture appetite changes or growth suppression. Across ADHD interventions, analyses indicated an effect on appetite suppression in studies reporting continuous outcomes (SMD 0.41; CI 0.01, 0.82; 11 studies, n=1321). Heterogeneity was high (I-squared 90%). The type of intervention was one source of heterogeneity, as indicated in a meta-regression (p 0.02). There was no evidence of publication bias. Removing two high-risk-of-bias studies in a sensitivity analysis found a similar point estimate (SMD 0.46; CI −0.05, 0.97) and heterogeneity was not reduced. Across all ADHD interventions, ADHD treatment was associated with decreased appetite compared to control group participants (RR 2.77; CI 2.21, 3.46; 66 studies, n=9508). A large number of studies and participants contributed to the results, and while many individual interventions did not detect statistically significant effects for this rare event, the data aggregation across studies shows a statistically significant effect. Heterogeneity was not remarkable (I-squared 53%). We tested whether the intervention type explained some of the heterogeneity and found evidence that this was the case (p 0.002). It should be noted that adverse events generally were more systematically reported in drug studies, and this outcome in particular was usually only reported in studies evaluating a pharmacological component; hence the analysis of the source of heterogeneity should be interpreted with caution. There was some evidence of publication bias (Egger p 0.01, Begg p<0.001). The alternative estimate of the effect using the trim and fill method to account for unpublished studies was somewhat smaller (RR 2.21; CI 1.74, 2.80). We also conducted a sensitivity analysis removing nine high risk-of-bias studies; the resulting estimate suggested an even stronger effect (RR 3.01; CI 2.38, 3.80) and heterogeneity was reduced further.
5.2.8. Effects of ADHD Treatment on Number of Participants With Adverse Events
Several identified studies reported on the number of participants experiencing at least one adverse event. Across ADHD interventions, participants undergoing active ADHD treatment were more likely to report adverse events than control group participants (RR 1.26; CI 1.19, 1.33; 64 studies, n=9632). We did not detect notable heterogeneity in this analysis (I-squared 59%). An analysis of the intervention as a potential source of heterogeneity indicated that the type of intervention was associated with the reported effect estimate (p<0.0001). There was no evidence of publication bias. Removing 11 high risk-of-bias studies in a sensitivity analysis did result in a similar point estimate (RR 1.25; CI 1.18, 1.33) and heterogeneity estimates were unchanged.
5.3. Effects by Intervention
The identified interventions were very diverse and addressed ADHD treatment in very different ways. In addition, exploring heterogeneity across studies indicated that for several key outcomes the type of intervention that was evaluated is a key source explaining variation in effect estimates. Hence, we broadly differentiated different types of interventions:
- Combined pharmacological and youth-directed psychosocial treatment
- FDA-approved pharmacologic treatment
- Other pharmaceutical agents
- Youth-directed psychosocial treatment
- Cognitive training
- Neurofeedback
- Neurostimulation
- Physical exercise
- Nutrition and supplements
- Complementary, alternative, and integrative medicine
- Parent support
- School interventions
- Provider intervention
These intervention categories provide broad clusters for analyses. The scope of each intervention category is described in detail in each intervention section. In addition to categorizing the type of intervention, we noted whether the intervention was tested as an ‘add-on,’ i.e., it was given in addition to and concurrently with stimulant medication. In these studies, the intervention as well as the control group received stimulants while the intervention group was given an additional intervention component.
The following provides an overview of the available studies for each intervention category, together with a summary of the effects of the interventions on outcomes. Each section starts broad, addressing a broad question associated with the intervention class, such as whether medication can improve outcomes at all compared to a concurrent control group or an active comparator. Each section then explores empirically whether subgroups of interventions were associated with different treatment effects. Finally, each study addresses a unique research question with a relatively unique intervention. Throughout the report, forest plots show not only the results across studies, but document also the results of each individual study. The study ID (author, publication year, unique identifier) is shown in the list of included studies in the appendix together with the full citation for the main publication of the study. In addition, intervention characteristics for particularly successful interventions are described in more detail in the text. We also refer the reader to the appendix, where for each included study a narrative summary of the results for all key outcomes are documented in a comprehensive evidence table.
5.3.1. Combined Pharmacological and Youth-Directed Psychosocial Treatment
We identified 11 eligible treatment studies that evaluated a combination of pharmacological intervention and youth-facing nonpharmacological psychosocial therapy.107, 201, 216, 275, 343, 357, 474, 497, 560, 589, 597 The behavioral or psychological treatment had to be directed at the participating children and adolescents in order to be included in this treatment category. Studies assessing the effect of parental training in combination with medication are reported in the parent intervention section. The earliest identified set of studies were those published from the National Institute of Mental Health Multimodal Treatment Study of Children with ADHD (MTA), which dates to 1999343 that has been reported thus far in 73 articles, as shown in the evidence table. Studies were published in six countries but half of the identified combined pharmacological and behavioral studies were conducted in the United States.151, 275, 343, 497, 1163
The populations studied were predominately males and the proportion of girls ranged from seven to 26. Studies included children and adolescents between the ages of five and 18. Evidence of intellectual disability (i.e., full-scale IQ < 70) was exclusionary in all studies, and most studies required full-scale IQ scores of 80 or higher. Half of the studies allowed participants to be included if they had prior exposure to stimulant treatment for ADHD, whereas the remaining studies required participants to be stimulant naïve, or else it was unclear what their inclusion criteria were regarding prior treatment with stimulant medication. For studies that distinguished between ADHD presentations (i.e., ADHD-combined type, ADHD-inattentive type, and ADHD-hyperactive/impulsive type), the most prevalent type (ranging from 54%201 to 88%343 of the ADHD participants) was the ADHD-combined presentation. In most studies, children were allowed to have common co-occurring conditions such as oppositional defiant disorder, conduct disorder, or dyslexia/learning disorder, but more severe neurodevelopmental conditions such as autism were exclusionary in this subarea of studies. Most studies reported at least some general information regarding the racial/ethnic makeup of their sample; on average, children of Caucasian/European ancestry comprised two thirds of sample makeup, a third were Hispanic or Latino, and a smaller percentage were African American.
The pharmacological treatment components employed in the studies were predominantly short- or long-acting stimulants (such as methylphenidate and amphetamine)201, 257, 343, 497 or else the non-stimulant medication atomoxetine.216 Behavioral treatment components varied in approach and complexity. Four studies evaluated cognitive behavioral therapy201, 216, 497, 560 and three described multi-modal psychosocial treatments.107, 333, 343 One study each evaluated a behavioral and social skills class;589 one a complex intervention with brief early intervention, parent component, and cognitive behavioral therapy for adolescents,275 one a humanistic intervention,474 and one a solution-focused approach.357 Studies compared most frequently to pharmacology treatment alone, rather than no treatment or placebo. These “add-on” trials, where one group receives an additional intervention, predominantly evaluated whether the combination treatment was superior to the medication intervention that all participants received.
Studies reported a variety of often study-specific outcomes. In terms of pre-specified key outcomes, symptom scores were most frequently reported.
Three studies reported on changes in a specific problem behavior, but they reported different effect estimates and could not be combined into a meaningful summary estimate shown by the large confidence interval; none detected statistically significant difference between the intervention and a control group (SMD −1.28; CI −7.56, 5.00; 3 studies, n=329).107, 275, 343 Two of the identified studies reported long-term effects, but they reported very small effects with conflicting direction of effects (SMD 0.04; CI −2.15, 2.20).107, 343
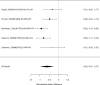
Studies reporting on broadband measures are shown in Figure 20.
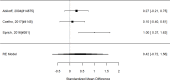
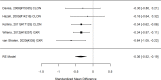
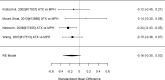
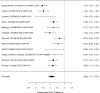
Figure 20
Effects of combined pharmacological and youth-directed psychosocial treatment on broadband measures (SMD). Notes: ADHD = attention deficit hyperactivity disorder, RE = random effects, SMD = Standardized Mean Difference
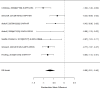
Across studies, we found no systematic difference between intervention and control groups (SMD 0.42; CI −0.72, 1.56; 3 studies, n=171), but it should be noted that all studies included in this analysis compared to the medication component of the combined intervention (i.e., control participants received one of the two intervention components). The included studies evaluated different interventions (multimodal psychosocial treatment plus methylphenidate;107 group cognitive behavioral therapy (CBT) plus methylphenidate;201 and individual CBT plus FDA-approved medication560) and compared to medication alone. The analysis detected some heterogeneity (I-squared 62%). There was no indication of publication bias. All three studies were judged to be high risk of bias. A study reporting on a categorical outcome also found no difference between studies (RR 0.85; CI 0.54, 1.36; 1 study, n=227).497 Only one of the studies reported a long-term outcome; the effect of the intervention was not statistically significant (SMD 0.27; CI −0.21, 0.75).107
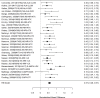
Studies reporting on ADHD symptom scales are shown in the next forest plot (Figure 21).

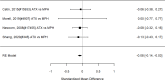
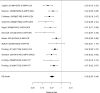
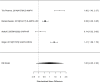
Figure 21
Effects of combined pharmacological and youth-directed psychosocial treatment on ADHD symptoms (SMD). Notes: ADHD = attention deficit hyperactivity disorder, RE = random effects, SMD = Standardized Mean Difference
Studies did not identify a statistically significant effect of superiority of the combined pharmacological and psychological treatment versus control (SMD −0.36; CI −0.73, 0.01; 7 studies, n=841). However, the pooled effect was very close to being statistically significant and several of the individual studies reported positive (although not necessarily statistically significant) effects. The strongest effects were reported for a behavioral and social skills functioning class for children and their parents,589 and for cognitive behavioral therapy with adolescents560 in another study. Additionally, when interpreting the results of combined pharmacological and behavioral interventions, it should be noted that the control groups against which the intervention is compared consisted of groups that received the pharmacological intervention component alone rather than no intervention. Hence, the analysis was typically a type of comparative effectiveness analysis rather than a pure effectiveness analysis against a passive comparator. There was some indication of statistical heterogeneity (I-squared 76%). The analysis did not detect publication bias. Removing four high-risk of bias studies in a sensitivity analysis did not result in a different effect estimate, and the effect was also not statistically significant (SMD −0.35; CI −1.80, 1.10). Only the MTA study reported on a long-term outcome (36 months, SMD −0.11; CI −0.36, 0.15).343 The study did also not detect a difference between the combined and medication alone group at post-intervention for ADHD symptoms (inattention teacher ratings at 14 months, SMD 0.01; CI −0.23, 0.26) and using this alternative estimate for the MTA study did also not detect a statistically significant effect of the combined treatment across all studies (SMD −0.34; CI −0.73, 0.04). The next forest plot (Figure 22) shows studies reporting on a categorical symptom assessment.
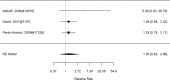
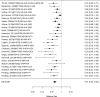
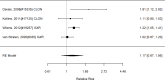
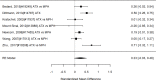
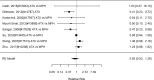
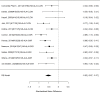
Figure 22
Effects of combined pharmacological and youth-directed psychosocial treatment on ADHD symptoms (RR). Notes: RE = random effects, RR = Relative Risk
Studies did not identify a statistically significant treatment effect in the categorical outcome either (RR 1.35; CI 0.63, 2.86; 3 studies, n=155) that would suggest superiority of the combined treatment compared to medication alone. There was no indication of heterogeneity in this small set of studies and further analyses were not possible due to the small number of studies. Of these, two studies reported on outcomes of 12 months or more; because effect estimates differed widely, no meaningful summary estimate could be derived (RR 1.72; CI 0.00, 2038).107, 474
Studies reporting on functional impairment reported conflicting results and no meaningful summary estimate could be derived due to wide confidence intervals (SMD 0.02; CI −2.54, 2.56; 2 studies, n=261). Heterogeneity was negligible, but the number of studies was small and no further analyses could be conducted. The estimate included the MTA study that reported a long-term effect of the intervention (36 months, SMD 0.11; CI −0.14, 0.36, 14 months SMD −0.05; CI −0.38, 0.27).
The MTA study also reported on an academic performance measure and did not detect a statistically significant effect (36-months SMD −0.12; CI −0.37, 0.13; 1 study, n=243; 14 months SMD −0.10; CI −0.34, 0.14).343 No other study reported on academic performance. We did not identify studies reporting on treatment satisfaction.
One study reporting on appetite suppression that reported sufficient data for effect size calculation found no difference between groups, where both received atomoxetine (RR 0.93; CI 0.29, 3.03; 1 study, n=29).216 The MTA reported that after 14 months, children treated with methylphenidate had gained less height and less weight (−1.23 cm per year and −2.48 kg per year) than untreated children669 and follow up into young adulthood within naturalistic subgroups of ADHD cases showed that extended use of medication was associated with suppression of adult height.343
One identified study reported on the number of participants experiencing adverse events and documented one hospitalization, but the outcome was considered unrelated to the study and there was no systematic difference between groups (RR 3.00; CI 0.13, 68.57; 1 study, n=32).275
5.3.1.1. Combined Pharmacological and Youth-Directed Psychosocial Treatment Summary of Findings
Table 12 shows the findings for all key outcomes of interest, together with the number of studies reporting on the outcome and study identifiers. The findings column shows the pooled estimate across studies. Not all studies reporting on the outcomes of interest contributed to each pooled estimate (e.g., because they did not report sufficient detail to allow effect size calculations). Results of individual studies are documented in the evidence table in the appendix and, for each study and outcome, results are summarized in a narrative summary (including results for the key outcomes that provided insufficient detail for effect size calculations).
Table 12
KQ2 summary of findings and strength of combined pharmacological and youth-directed psychosocial treatment.
The summary of findings table above generally shows little support that a treatment modality comprising combined medication and youth-directed psychosocial treatment as superior to control groups receiving mono-therapy (typically medication alone). For multiple outcomes we found very few or no studies to determine intervention effects. We downgraded the strength of evidence for functional impairment, academic performance, and adverse events to insufficient due to study limitation and inconsistency (downgraded by 2 given that consistency could not be determined as only one study has reported on the outcome to date). The strength of evidence for symptom improvement was downgraded for imprecision (the result was not statistically significant but the confidence interval was very close to including a positive effect for the combined intervention).
5.3.2. FDA-Approved Pharmacologic Treatment
We identified 106 studies evaluating a pharmacological intervention approved by the FDA for the treatment of ADHD.108, 109, 118, 127, 131–133, 137, 144, 145, 154, 161, 164, 175, 193–196, 202, 205, 207, 217, 220, 224–226, 235, 247–250, 270–273, 281, 286, 288, 289, 292, 305, 306, 317, 321, 326, 337, 341, 348, 361, 373, 374, 376, 378, 380, 381, 383, 387, 414, 418, 419, 425, 431, 432, 442, 452–455, 459–461, 481, 504, 511, 512, 525, 526, 538–540, 554–557, 561, 568, 573, 575, 588, 598, 604, 608, 610–612, 616–619, 621–623, 626, 634, 645 Although studies from 1980 were eligible, the earliest studies meeting inclusion criteria were published in 1995.540 Evaluations were published in 16 different countries (and some were conducted in multiple countries) but 60 percent of the research was U.S.-based. Although the reported percent of female participants ranged from under one percent to 56 percent, samples were predominantly male. The age minimum varied, but across all identified studies, only four studies included young children three to five years old.109, 194, 271, 378 Studies varied in whether they required participants to be drug naïve at study beginning, while others allowed concomitant medication even during the study. The identified studies included some that explicitly tested adjunctive medication to augment stimulant treatment.104, 107, 257, 373, 474, 488, 598, 622 Studies included different presentations of ADHD. Where reported, the combined presentation was most common in studies, on average representing two thirds of the sample. While ADHD participants with co-occurring disorders were not excluded from most of the studies, only a few studies purposely included specific co-occurring disorders, including oppositional defiant disorder or conduct disorder,207, 220, 226, 432, 623 Tourette syndrome or tic disorder,118, 380, 540, 556 or learning disabilities.526, 538 Demographics were often not reported, but where studies described a breakdown by race or ethnicity, on average about 75 percent of children were White, about 15 percent Black, less than ten percent Hispanic, and about one percent were described as Asian.
Studies evaluated stimulants and non-stimulants, either alone or in combination. Interventions included the stimulant classes methylphenidate and amphetamine, and the non-stimulant classes norepinephrine reuptake inhibitor (NRI) and alpha agonists. Studies evaluated different methylphenidate hydrochloride formulations, including immediate, extended, and multilayer release formulations, methylphenidate osmotic-release oral system, and methylphenidate transdermal patch. Studies evaluated different amphetamine formulations, including amphetamine and dextroamphetamine mixed salts and lisdexamfetamine dimesylate. The NRI studies evaluated atomoxetine or extended-release viloxazine. The alpha agonist studies evaluated extended-release clonidine or extended-release guanfacine. The most commonly evaluated single medication was atomoxetine in the identified studies.
Of the identified studies, the majority reported on the comparison to a control group not receiving the evaluated pharmacological treatment, and the large majority used a placebo to blind participants to the intervention allocation. Several studies provided methylphenidate as base treatment for the intervention and control group. Half of the identified studies reported on the effects of an alternative intervention, for example a different dose of the same medication or a different medication.
The following shows the effects of FDA-approved medication as a group of interventions given that whether or not subjecting children to regular medication use is a key question for parents, regardless of the pharmacological composition of the specific medication. The section is followed by a comparative effectiveness section to determine whether there are systematic differences between medication combinations (stimulants plus non-stimulants), the medications categories (stimulant or non-stimulant), drug classes (methylphenidate, amphetamine, NRIs, and alpha agonists), or individual medications (e.g., methylphenidate hydrochloride extended release, amphetamine and dextroamphetamine mixed salts, atomoxetine, or clonidine etc.).
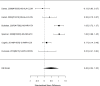
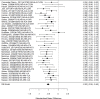
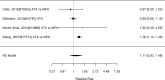
Studies most frequently reported on ADHD symptom scale scores. Studies that reported on a control group with sufficient detail to allow effect size calculations for individual behavior changes (not already captured in broadband or symptom score measures) are shown in Figure 23. The forest plot is ordered by broad category (non-stimulant or stimulant), drug class (methylphenidate, amphetamine, NRIs, and alpha agonists, followed by the specific drug evaluated in the study (e.g., guanfacine).
Across studies, pharmacological interventions were associated with significant improvements in individual problem behaviors (SMD −0.62; CI −0.97, −0.27; 5 studies, n=561). The minimum age in the included studies was six years old. There was little evidence of heterogeneity (I-squared 45%). There was no indication of publication bias and none of the RCTs were judged to be high risk of bias. We identified one study reporting on a categorical variable based on a behavior measure and providing sufficient detail to allow effect size computation. The identified study evaluated the alpha-agonist clonidine adjunctive to psychostimulant medication321); the study reported positive results (RR 0.36; CI 0.17, 0.78; 1 study, n=66).
Multiple studies reported on a broadband measure (see key outcome section) describing the children’s potential improvement on broader dimensions than specific ADHD symptoms, as shown in Figure 24.
Across studies, pharmacological treatment was associated with a systematic benefit on broadband scale assessments compared to control (SMD 0.57; CI 0.48, 0.67; 28 studies, n=4467). Only one study included children younger than six years old.109 Studies assessed different medication regimes but analyses detected little heterogeneity (I-squared 50%). Large effects were reported in studies evaluating lisdexamfetamine dimesylate,131 atomoxetine,248 methylphenidate,109 and extended-release guanfacine added to usual care stimulant therapy,598 respectively. There was no evidence of publication bias. Removing six high-risk-of-bias RCTs in a sensitivity analysis found a smaller but also significant effect estimate (SMD 0.53; CI 0.38, 0.69), indicating that the documented treatment effect is not mainly based on biased studies. Multiple studies reported on broadband scale as a categorial outcome (e.g., criteria for improvement met or not) as shown in Figure 25.
Across studies, results also indicated that pharmacological ADHD treatment was associated with a benefit in outcomes compared to control (RR 0.51; CI 0.43, 0.60; 25 studies, n=3959). Only two studies included children younger than 6 years old.109, 378 Analyses detected some heterogeneity (I-squared 75%). There was evidence of publication bias (Begg p<0.001, Egger p<0.001) and an alternative estimate using the trim and fill method suggested a somewhat smaller effect (RR 0.62; CI 0.52, 0.74). When excluding six high-risk-of-bias RCTs in a sensitivity analysis, effect estimates were similar to the original effect (RR 0.49; CI 0.40, 0.59) and heterogeneity was not reduced (I-squared 80%). All studies reported on less than 12 months follow up with the exception of one study; the study found a significant improvement (SMD 4.74; CI 4.36, 5.13).164
A large number of studies reported on symptom improvements. Standardized mean differences are shown in Figure 26.
Across studies, pharmacological interventions for ADHD were associated with a systematic reduction in ADHD symptom scale scores compared to control (SMD −0.61; CI −0.69, −0.52; 49 studies, n=7685). Only two studies included children younger than six years old.109, 378 There was some heterogeneity (I-squared 64%). Tests for publication bias were not statistically significant. Excluding nine high-risk-of-bias RCTs in a sensitivity analysis estimated similar symptom reductions, indicating that the result is not primarily driven by high-risk studies (SMD −0.60; CI −0.71, −0.49). Results for symptom measures used as categorical variables (e.g., number of improved children meeting a scale threshold) are shown in Figure 27.
Results across studies also indicated a significant benefit (RR 1.71, CI 1.33, 2.19; 13 studies, n=1918). None of the studies included children under six years of age. There was some evidence of heterogeneity (I-squared 69%). There was also some evidence of publication bias (Begg p 0.02, Egger p 0.01). Applying the trim and fill method for an alternative estimate, effects were smaller (RR 1.45; CI 1.11, 1.88). When removing high-risk of bias RCTs in a sensitivity analysis, the treatment effect was similar to the main analysis (RR 1.79, CI 1.40, 2.30) and heterogeneity was further reduced, indicating that methodological rigor of the studies was one source of heterogeneity. Only one of the studies reported on a long-term outcome; the effect was not statistically significant (SMD 0.04; CI −0.27, 0.35).164
Some of the identified studies reported on functional outcomes as shown in Figure 28.
Across studies, treatment was associated with a decrease in functional impairment (SMD 0.50; CI 0.05, 0.96; 10 studies, n=1703). Only one study included children younger than six years old.109 There was evidence of substantial heterogeneity (I-squared 93%). There was no evidence of publication bias. Excluding two high risk of bias RCTs in a sensitivity analysis did not change the effect (SMD 0.61; CI 0.05, 1.17) and heterogeneity was not reduced. We stratified studies by medication to determine whether the type of medication is a source of heterogeneity. There was some indication that heterogeneity was reduced in selected subgroups (amphetamines), but heterogeneity remained high in multiple subgroups and we did not identify broad treatment categories (stimulants, non-stimulants, the stimulant subtype amphetamines, NRIs, the NRI subtype atomoxetine) as a clear source of heterogeneity. The only study reporting a long-term effect was not statistically significant (SMD 0.02; CI −0.29, 0.33).164
We identified only one study that formally assessed treatment satisfaction for all study arms; it reported significant satisfaction with the alpha agonist treatment compared to placebo treatment (RR 0.47; CI 0.32, 0.68; 1 study, n=198).207
Only one study reported on academic performance; the study reported improvements in the methylphenidate compared to control group (SMD −1.37; CI −1.72, −1.03; 1 study, n=156) in the correct answers on the Permanent Product Measure of Performance.618
All studies reporting in sufficient detail on a continuous measure for appetite, weight or growth suppression that allowed us to compute measure-independent standardized mean differences are shown in Figure 29.
Across studies, pharmacological treatment indicated reduced appetite, but the effect was not statistically significant (SMD 0.48; CI −0.04, 1.00; 6 studies, n=605). There was evidence of heterogeneity (I-squared 82%). The analysis included stimulants and non-stimulants, but NRIs were only represented by atomoxetine and alpha agonists only by clonidine. Two atomoxetine studies reported a smaller increase in weight than children in the placebo group. Removing one high-risk-of-bias RCT in a sensitivity analysis did not change the finding (SMD 0.52; CI −0.13, 1.18) and heterogeneity was not reduced. We did not detect publication bias. A much larger number of studies reported on appetite suppression as a categorical measure (e.g., reported incidences per sample) indicating the number of patients reporting this adverse event as shown in Figure 30.
Across studies, pharmacological treatment was associated with a suppression in appetite compared to control groups (RR 3.51; CI 2.72, 4.51; 46 studies, n=7209). Only two studies included children under the age of six.194, 378 Heterogeneity was negligible (I-squared 41%). There was evidence of publication bias (Begg p 0.02, Egger p<0.002). An alternative treatment estimate using the trim and fill method suggested a somewhat smaller effect on appetite suppression (RR 2.66; CI 2.02; 3.50). When removing four high-risk-of-bias RCTs in a sensitivity analysis, effect estimates were similar to the main effect (RR 3.62; CI 2.77, 4.74). Only one of the studies evaluating appetite suppression reported on a long-term outcome, it indicated less weight increase compared to placebo (SMD 1.05; CI 0.72, 1.37).164
The number of participants experiencing any adverse event is documented in Figure 31.
Pharmacological interventions were associated with a higher risk of experiencing adverse events compared to control groups (RR 1.29; CI 1.23, 1.35; 41 studies, n=6926). None of the studies included children under the age of six. We detected only negligible heterogeneity (I-squared 47%). There was evidence of publication bias (Begg p 0.03, Egger p<0.001) and an alternative effect estimate using the trim and fill method suggested a smaller effect (RR 1.21; CI 1.15, 1.28). We also assessed in a sensitivity analysis whether results were mainly driven by high-risk-of-bias studies; estimates remained stable (RR 1.30; CI 1.23, 1.36) after excluding eight high-risk of bias RCTs and heterogeneity was reduced further. Only one of the identified studies reported a long-term effect; which showed more participants reporting adverse events in the intervention group compared to placebo (RR 1.22; CI 1.02, 1.47).164
5.3.2.1. FDA-Approved Pharmacologic ADHD Treatment Comparative Effects
We identified over 60 studies comparing pharmacological agents to an alternative treatment; however, comparators varied. Comparators were often different doses of the same medication, and some found a dose-response effect. For example, one study compared 200mg with 100mg of extended release viloxazine (an NRI) and reported improvement in both symptoms and functional impairment in both dosage groups, while the rate of children reporting decreased appetite was 7.5 percent in the 200mg group compared to 4.5 percent in the 100mg group.453 The evidence table in the appendix shows results for dose comparisons in detail.
The following documents results of direct comparisons within head-to-head trials, followed by indirect comparisons across studies where possible.
5.3.2.1.1. Combined Effects: Non-Stimulants Plus Stimulants Versus Stimulants Alone
Several studies evaluated the effect of an intervention in samples already receiving treatment for ADHD. Most often the ongoing intervention was described as stimulant treatment. Hence, the group of tested non-stimulant evaluation studies included studies where participants were already receiving stimulants and the new therapy was assessed as an adjunctive treatment. The stimulant medication would be taken by both the intervention and control group participants. We systematically identified studies that augmented usual care with an additional treatment, and we determined in a meta-regression whether this intervention-comparator combination affects the treatment effects. We were particularly interested in whether medication add-on trials reported systematically different results from other studies. This could be either a specific stimulant, such as methylphenidate, or stimulants not further described. Often the stimulant dose was either not known, or it varied by participant based on the usual care arrangement. Most analyses for the outcomes of interest were not statistically significant: behavior (p 0.33), broadband measures (continuous p 0.81 categorical p 0.14), appetite suppression (continuous p 0.28, categorical p 0.24), participants reporting adverse events (p 0.14). For other outcomes, there were insufficient studies for the comparison (functional impairment, treatment satisfaction, academic performance). However, for ADHD symptoms using continuous outcome variables, there was indication that the effect estimate depended to some extent on whether participants were already receiving stimulants (p 0.048). The effect was not found for categorical outcome measures (p 0.77). The following analyses report on the subgroup of studies that augmented stimulant medication with a non-stimulant.
We identified one study that compared clonidine plus stimulants versus stimulants alone and that reported on a problem behavior; the study favored the combination (RR 0.36; CI 0.17, 0.78; 1 study, n=66).321
Two studies reported on a continuous broadband measure, but since the reported effects varied, no meaningful summary for the augmentation could be determined (SMD 0.52; CI −1.26, 2.30; 2 studies, n=292).373, 598 However, a single study found that adjuvant treatment with guanfacine was associated with a statistically significantly greater number of improved participants (RR 0.80; CI 0.68, 0.95; 1 study, n=303).622
Results for ADHD symptoms for the subgroup of non-stimulants are shown in Figure 32.
Across studies, non-stimulant augmentation (all studies used alpha agonists) of stimulants found a statistically significant effect for ADHD symptoms across studies (SMD −0.26; CI −0,52, −0.19; 5 studies, n=724). Only one study evaluated an add-on trial reporting on a categorical symptom outcome; the study did not detect a systematic difference (RR 2.04; CI 0.82, 5.06; 1 study, n=66).321
This subgroup of studies did not assess functional outcomes, treatment satisfaction, or academic performance. And although some of the studies reported on appetite suppression, the two studies that reported on a continuous outcome reported conflicting results and no meaningful summary estimate could be derived (SMD 0.13; CI −03.12, 3.39; 2 studies, n=128).217, 321 The single study reporting a categorical outcome did not detect a statistically significant difference between treatment arms (RR 1.52; CI 0.56, 4.19; 1 study, n=303).622
Figure 33 shows the effects of non-stimulants plus stimulants versus stimulants alone on the number of participants with adverse events.
Across studies, we detected no systematically different effect of the combination treatment on appetite suppression compared to stimulant alone (RR 1.17; CI 0.87, 1.56; 4 studies, n=657).
5.3.2.1.2. Medication Category Comparison: Non-Stimulants Versus Stimulants
We also differentiated the included studies into those assessing the effects of non-stimulants and stimulant medications. We reviewed direct comparisons of a non-stimulant with a stimulant as well as meta-regressions using indirect comparisons. The indirect comparisons aimed to detect whether studies comparing non-stimulants versus control reported statistically significantly different results from stimulants versus control.
Non-stimulants versus stimulants in direct, head-to-head comparisons within identified studies for individual problem behaviors are shown in Figure 34.
Across comparative effectiveness studies, non-stimulants (all NRIs) were slightly but statistically significantly associated with more reductions in individual problem behavior compared to stimulants (SMD −0.08; CI −0.14, −0.03; 4 studies, n=608); all studies compared atomoxetine versus methylphenidate specifically rather than the full range of non-stimulant or stimulant medications. None of the studies included children under the age of six. The analysis did not detect heterogeneity or evidence of publication bias. However, removing all high-risk of bias studies left only two studies, which individually did not detect a systematic difference between atomoxetine versus methylphenidate (SMD −0.10; CI −0.40, 0.20). There were insufficient studies reporting on the outcome for indirect comparisons between non-stimulant and stimulant studies. Given the difference between medications shown in the head-to-head trials, Figure 35 reports a subgroup analysis for non-stimulants on problem behavior.
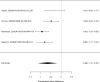
Figure 35
Subgroup analysis: Non-stimulants versus control on problem behavior (SMD). Notes: NRI = norepinephrine reuptake inhibitors, NS-NRI-ATX = atomoxetine, NS-ALA-CLON = clonidine, RE = random effects, SMD = standardized mean difference
In the subgroup of non-stimulant studies, treatment was associated with a reduction in problem behavior compared to placebo (SMD −0.66; CI −1.10, −0.22; 4 studies, n=523). However, only atomoxetine, one of the two approved NRIs for the treatment of ADHD, and clonidine, one of two approved alpha agonists, contributed to the analysis. We identified only one study that compared stimulants alone to a control group; the study did not detect a systematic difference between immediate release methylphenidate and placebo (SMD 0.31; CI −0.33, 0.95; n=91).224
Results for broadband measures in the comparison of non-stimulants versus stimulants are shown in Figure 36; all studies compared atomoxetine with methylphenidate medications.
Across studies, we did not detect a systematic difference between stimulants and non-stimulants for continuous broadband measure outcomes (SMD −0.16; CI −0.35, 0.03; 4 studies, n=1080); all studies compared the NRI atomoxetine versus methylphenidate medications.376, 460, 539, 604 Other stimulants (amphetamine) and non-stimulants (alpha agonists) could not be added to the analysis, due to lack of studies. We did not detect heterogeneity or evidence of publication bias in this analysis. Removing all high-risk of bias studies left only one study that reported a similar effect estimate (SMD −0.15; CI −0.37, 0.06).604 We also assessed in indirect comparisons whether the subgroup of studies evaluating non-stimulants versus studies evaluating stimulants reported different effect sizes (both compare the intervention against a control group, rather than comparing the two drug classes directly). We did not detect differences for continuous outcomes in this analysis (p 0.88). We identified only one study that reported on a categorical assessment of a broadband impression; the study found no difference between non-stimulants and stimulants (RR 1.01; CI 0.75, 1.37; 1 study, n=237); the study compared the NRI atomoxetine versus methylphenidate medication specifically.568 However, a meta-regression for categorical broadband measures indicated a statistically significant difference between results reported in non-stimulant versus stimulant studies (p 0.0002). Figure 37 shows the subgroup analysis results for non-stimulants.
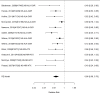
Figure 37
Subgroup analysis: Non-stimulants versus control on broadband measures (RR). Notes: NS-NRI-ATX = atomoxetine, NS-ALA-GXR = guanfacine, RE = random effects, RR = risk ratio
In the subgroup of non-stimulant studies, treatment was associated with a reduction in broadband measures, but the effect was smaller than for stimulants (RR 0.66; CI 0.58, 0.76; 12 studies, n=2312). Only two out of four FDA-approved non-stimulant medications (atomoxetine, guanfacine) contributed to the analysis. Only one of the studies in this subgroup included children under the age of 6.378 The subgroup analysis of stimulant studies is shown in Figure 38.
The effect estimate for stimulant studies showed a clear effect for individual studies and across studies in this medication subgroup (RR 0.38; CI 0.30, 0.48; 12 studies, n=1582). Only one study included children younger than six years old.109
A large number of studies reported on ADHD symptoms, and we identified a number of head-to-head comparisons. The analysis comparing non-stimulants versus stimulants for ADHD symptoms is shown in Figure 39.
Although more studies favored stimulants, across studies, we did not detect a systematic difference between non-stimulants (all NRI) versus stimulants (different methylphenidate medications in all but one case) in direct comparisons for ADHD symptoms (SMD 0.23; CI −0.03, 0.49; 7 studies, n=1611). We detected some heterogeneity (I-squared 69%) in this analysis. There was no evidence of publication bias. Removing all high-risk of bias studies left three studies that also found no systematic difference between interventions (SMD 0.28; CI −0.54, 1.10). However, we also analyzed whether indirect comparisons between non-stimulant versus stimulant studies indicate systematic differences, and we found a statistically significant difference (p 0.0002). The effect estimates for the subgroups are documented in the following section. Figure 40 shows the subgroup analysis for non-stimulants reporting on ADHD symptoms.
In the subgroup of non-stimulant studies, results were associated with a reduction in ADHD symptoms measured as a continuous variable (SMD −0.52; CI −0.59, −0.46; 37 studies, n=6065). Only one study included children younger than six years old.378 Results for the subgroup of stimulant studies on ADHD symptoms are shown in Figure 41.
In the subgroup of stimulant studies, treatment was associated with a substantial reduction in ADHD symptoms (SMD −0.88; CI −1.13, −0.63; 12 studies, n=1620). Only one study included children younger than six years old.109 None of the direct, head-to-head trials reported on symptom improvement as a categorical measure (e.g., treatment response vs not). An indirect comparison suggested that non-stimulant versus stimulant studies report statistically significantly different results for categorical ADHD symptom measures (p 0.02). The subgroups are shown separately in Figure 42 and Figure 43.

Figure 42
Subgroup analysis: Non-stimulants versus control on ADHD symptoms (RR). Notes: ADHD = attention deficit hyperactivity disorder, NS-NS-NRI-ATX atomoxetine, NS-ALA-CLON clonidine, NS-NRI-VLX viloxazine, RE = random effects, RR = relative risk
In the subgroup of non-stimulant studies, we found a clear treatment effect on ADHD symptoms (RR 1.51; CI 1.26, 1.81; 12 studies, n=1765). However, only three non-stimulant studies contributed to the analysis (atomoxetine, viloxazine, and clonidine). None of the studies included children under the age of six. However, the effect was not as pronounced as in the single stimulant study that was identified (evaluating lisdexamfetamine dimesylate), which reported a very large treatment effect versus control (RR 4.28; CI 2.49, 7.35; 1 study, n=153).202
We did not identify studies reporting on functional impairment in a head-to-head comparison. Indirect analyses comparing non-stimulant versus stimulant studies showed a statistically significant result (p 0.04). Subgroup analyses are shown in Figure 43.
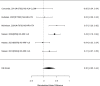
Figure 43
Subgroup analysis: Non-stimulants versus control on functional impairment (SMD). Notes: NS-NS-NRI-ATX = atomoxetine, NS-ALA-CLON = clonidine, NS-NRI-VLX = viloxazine, RE = random effects, SMD = Standardized Mean Difference
In the subgroup of non-stimulant studies, treatment was associated with a small but not statistically significant improvement in functional impairment (SMD 0.20; CI −0.05, 0.44; 6 studies, n=1163). However, only atomoxetine, viloxazine, and clonidine studies contributed to the analysis. None of the studies included children under the age of six. The equivalent analysis for stimulant studies is shown in Figure 44.
In the subgroup of stimulant studies, treatment was not associated with statistically significant improvement in functional impairment across studies (SMD 1.00; CI −0.25, 2.26; 4 studies, n=540). Only one study included children younger than 6 years old.109
There were insufficient studies for analyses regarding treatment satisfaction as well as academic performance. Both direct and indirect comparisons could not be analyzed due to the small number of identified studies.
Results for direct comparisons between non-stimulants and stimulants for appetite suppression are shown in Figure 45.
Across studies, we found no systematic difference between non-stimulant (all identified studies evaluated the NRI atomoxetine) versus stimulants (RR 0.82; CI 0.53, 1.26; 8 studies, n=1463). No alpha agonists or NRIs other than atomoxetine contributed to this analysis. There continued to be heterogeneity (I-squared 78%). There was no evidence of publication bias. Removing high-risk of bias studies in a sensitivity analysis left only two studies; results remained not statistically significantly different between interventions (RR 1.34: CI 0.51, 3.52). When restricting the comparator to methylphenidate to determine whether the comparator is a source of heterogeneity, we found no systematic difference between NRI and methylphenidate medication interventions either and heterogeneity was reduced, but in this subset, all seven studies compared atomoxetine versus methylphenidate medications (RR 0.98; CI 0.67, 1.44; I-squared 58%). Results varied, sometimes favoring the NRI atomoxetine, sometimes the methylphenidate medications and across studies, no systematic difference was detected. Publication bias was not detected. An indirect comparison did not detect systematic differences between non-stimulant and stimulant studies for appetite suppression (p 0.31).
The comparative studies reporting sufficient detail to compute effect sizes for the number of participants with adverse events is shown in Figure 46.
Across studies, we found no systematic difference between non-stimulant (all identified studies were the NRI atomoxetine) versus stimulant interventions for the number of participants reporting adverse events (RR 1.11; CI 0.90, 1.37; 4 studies, n=756). There was some indication of heterogeneity (I-squared 63%). There was no evidence of publication bias. Removing high-risk of bias studies left one study comparing the NRI atomoxetine with methylphenidate (not further specified); the study favored stimulants (RR 1.28; CI 1.14, 1.45).604 We also evaluated in indirect comparisons across studies whether non-stimulant and stimulant studies vary systematically in effect size reporting. However, we did not detect an effect (p 0.12).
5.3.2.1.3. Stimulant Comparisons: Amphetamine Versus Methylphenidate
A small number of included studies compared amphetamine and methylphenidate in direct, head-to-head comparisons.
We did not identify any studies reporting on individual behaviors for a direct comparison of amphetamine and methylphenidate and indirect comparisons across studies also had insufficient number of studies for analyses for continuous as well as categorical outcomes.
A single study reported on a broadband measure and found more positive change in lisdexamfetamine dimesylate (an amphetamine) versus osmotic-release oral system methylphenidate (SMD 0.29; CI 0.02, 0.56; 1 study, n=211).131 Indirect comparisons across studies did not detect a systematic difference between amphetamine and methylphenidate studies (continuous outcomes p 0.97, categorical outcomes p 0.80). Figure 47 shows the results for the subgroup of amphetamine stimulants separately from those of methylphenidate.

Figure 47
Subgroup analysis: Amphetamine versus control on broadband measures (SMD). Notes: S-AMPH-LDX = lisdexamfetamine dimesylate, S-AMPH-MAS = mixed amphetamines salts, RE = random effects, SMD = standardized mean difference
Although all identified amphetamine studies in this subgroup reported positive effects, estimates varied and the pooled effect was not statistically significant (SMD 0.68; CI −0.72, 2.08; 3 studies, n=561). The analysis suggested substantial heterogeneity despite the small number of studies (I-squared 92%). There was no evidence of publication bias. None of the studies was determined to be high-risk of bias. The equivalent subgroup analysis for the stimulant methylphenidate is shown in Figure 48.

Figure 48
Subgroup analysis: Methylphenidate versus control on broadband measures (SMD). Notes: S-MPH-IR = immediate release methylphenidate, S-MPH-TP = transdermal patch methylphenidate, RE = random effects, SMD = standardized mean difference
The methylphenidate studies that compared to a passive control showed positive effects on broadband measures (SMD 0.66; 0.04, 1.28; 2 studies, n=302).
The single direct comparison study also reported better ADHD symptom control with the amphetamine lisdexamfetamine dimesylate versus osmotic-release oral system methylphenidate (SMD −0.46; CI −0.73, −0.19; 1 study, n=222).131 Indirect comparisons detected a statistically significant difference across studies for the continuous outcome analysis (p 0.02). Figure 49 shows the results separately for the two stimulant subgroups given that one study found a difference in reported effects in a head-to-head comparison of the two types of stimulants.
In the subgroup of amphetamine studies, we found a significant effect of treatment (SMD −1.16; CI −1.64, −0.67; 5 studies, n=757). None of the studies included children under the age of 6. The subgroup analysis results for methylphenidate studies are shown in Figure 50.
In the subgroup of methylphenidate studies, we found a significant treatment effect, but effect estimates were smaller (SMD −0.68; CI −0.91, −0.46; 7 studies, n=863). Only one study included children younger than 6 years old.109 Indirect comparisons between amphetamine and methylphenidate using categorical data were not statistically significant (p 0.57).
There was no statistically significant difference in functional impairment in a head-to-head comparison of the two stimulants (SMD 0.16; CI −0.11, 0.43; 1 study, n=211).131 The indirect comparison across studies did also not detect a systematic difference (p 0.68).
We identified no studies that reported on treatment satisfaction or academic performance in direct head-to-head comparisons and there were insufficient data for indirect analyses.
Results for direct comparisons between amphetamine and methylphenidate on the outcome appetite suppression are shown in Figure 51.
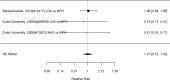
Figure 51
Comparison: Amphetamine versus methylphenidate on appetite suppression (RR). Notes: LDX = lisdexamfetamine dimesylate, MPH = methylphenidate, RE = random effects, RR = relative risk
The two studies reporting on appetite suppression did not find a difference between the amphetamine lisdexamfetamine dimesylate versus osmotic-release oral system methylphenidate, lisdexamfetamine dimesylate versus methylphenidate transdermal system, or mixed amphetamines salts versus osmotic-release oral system methylphenidate (RR 1.01; CI 0.72, 1.42; 3 comparisons, n=414). Similarly, indirect comparisons across studies did also not detect a statistically significant difference between the two stimulant classes for the categorical outcome analysis (p 0.08). Although the continuous outcome analysis was borderline statistically significant (p 0.05), only one study each contributed to the analysis. Both studies compared to placebo and none found a statistically significant difference between study arms (amphetamine SMD 0.17; CI −0.14, 0.48; 1 study, n=157; methylphenidate SMD 0.22; CI −0.41, 0.84; 1 study, n=40).202, 383
One study documenting the number of participants reporting adverse event found no statistically significant difference between stimulant classes (RR 1.11; CI 0.93, 1.33; 1 study, n=222); the study compared lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate.131 Similarly, indirect comparisons did also not detect a difference between amphetamines and methylphenidate regarding the number of participants reporting adverse events (p 0.35).
5.3.2.1.4. Non-Stimulant Comparisons: NRIs Versus Alpha Agonists
We identified a study directly comparing an alpha agonist (guanfacine) with an NRI (atomoxetine) in a head-to-head trial, but the study did not report on problem behaviors.326 In indirect comparisons, there were no differences for problem behaviors (p 0.31).
The guanfacine versus atomoxetine study detected no difference (RR 0.84; CI 0.68, 1.04; 1 study, n=226) for a categorical broadband measure (number of improved patients per Clinical Global Impression [CGI]).326 Indirect comparisons across studies also did not identify a systematic difference between NRIs and alpha agonists for broadband measures (continuous p 0.41, categorical p 0.19).
The same identified study comparing guanfacine with atomoxetine326 found that ADHD symptom improvement favored guanfacine over atomoxetine (SMD −0.47; CI −0.73, −0.2; 1 study, n=226). Indirect comparisons, however, did not suggest that alpha agonists systematically report different estimates for ADHD symptoms (continuous p 0.90, categorical p 0.57).
The following shows the subgroup results for NRI studies versus control separately for ADHD symptoms, given that a direct comparison of guanfacine versus atomoxetine study found a difference in effects (Figure 52).
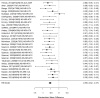
Figure 52
Subgroup analysis: NRIs versus control on ADHD symptoms (SMD). Notes: ADHD = attention deficit hyperactivity disorder, NS-NRI-GXR/NS-NRI-ATX = atomoxetine, NS-NRI-VLX = viloxazine, RE = random effects, SMD = standardized mean difference
In the subgroup of NRI studies, we found a clear effect on ADHD symptoms (SMD −0.55; CI −0.62, −0.47; 28 studies, n=4493). Only one study included children younger than 6 years old.378 The equivalent analysis for the subgroup of alpha agonist studies is shown in Figure 53.
In the smaller subgroup of alpha agonist studies, we also found a clear effect on ADHD symptoms (SMD −0.52; CI −0.67, −0.37; 11 studies, n=1885). It should be noted that the small difference between NRI versus control and alpha agonists versus control effect estimates was not statistically significant and is therefore indistinguishable from chance. None of the studies in this subgroup reported on children younger than 6 years of age.
Indirect comparisons across studies did not suggest a systematic difference in effects reported by NRI versus alpha agonist studies on functional impairment (p 0.46) and we found no head-to-head comparison between NRI and alpha agonist studies. Effects for treatment satisfaction and academic performance could not be evaluated in direct or indirect analyses due to lack of data.
The only identified study that reported a direct comparison between alpha agonists and NRIs found statistically significantly fewer instances of decreased appetite for guanfacine versus atomoxetine (RR 0.48; CI 0.27, 0.83; 1 study, n=226).326 Similarly, indirect comparisons indicated a significant difference between NRIs and alpha agonists for the outcome appetite suppression (categorical p 0.01). Subgroup results for appetite suppression are shown in Figure 54.

Figure 54
Subgroup analysis: NRIs versus control on appetite suppression (RR). Notes: NRI = norepinephrine reuptake inhibitors, NS-NRI-GXR/NS-NRI-ATX = atomoxetine, NS-NRI-VLX = viloxazine, RE = random effects, RR = relative risk
In the subgroup of NRI studies, we found a substantially increased risk of appetite suppression (RR 3.23; CI 2.40, 4.34; 27 studies, n=4176). Only one study included children younger than six years old.378 The equivalent analysis for the subgroup of alpha agonist studies is shown in Figure 55.
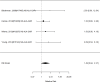
Figure 55
Subgroup analysis: Alpha agonists (all guanfacine) versus control on appetite suppression (RR). Notes: NS-ALA-GXR = guanfacine, RE = random effects, RR = relative risk
Unlike in the NRI studies, in the subgroup of alpha agonist (all guanfacine) studies, no statistically significant effect of appetite suppression was detected because confidence intervals were wider in this small subgroup (RR 1.49; CI 0.94, 2.37; 4 studies; n=919). Only guanfacine evaluations contributed to this result as no clonidine study reported on the outcome.
The one identified study that reported a direct comparison between NRIs and alpha agonists found no differences in the number of patients experiencing adverse events (RR 1.14; CI 0.97, 1.34; 1 study, n=226) between the interventions; the study compared guanfacine to atomoxetine, specifically.326 Potential differential effects for the number of participants reporting adverse events were not statistically significant in indirect comparisons across non-stimulants (p 0.06).
5.3.2.1.5. Drug Class Comparison: Methylphenidate Versus Amphetamine Versus NRIs Versus Alpha Agonists
The review identified over 100 studies evaluating dozens of FDA-approved medication treatments for ADHD. In addition to differentiating between stimulants and non-stimulants, we also tried to determine whether there are systematic differences between the four drug classes methylphenidate (stimulant), amphetamine (stimulant), NRI (non-stimulant), and alpha agonist (non-stimulant). A meta-regression across studies evaluated whether the drug class is associated with effect sizes.
For behavior outcomes, indirect comparisons did not detect a statistically significant difference in effect sizes (p 0.42).
Indirect analyses for the outcome broadband measures, however, indicated differences between intervention class across studies (p 0.002). Specifically, the analysis suggested that amphetamine studies (p<0.001) and methylphenidate studies (p<0.001) reported larger effects than alpha agonist studies. The subgroup of amphetamine studies reported the largest effect, but estimates varied across studies, and the pooled effect was not statistically significant in this small subgroup (SMD 0.68; CI −0.72, 2.08; 3 studies, n=561). The subgroup of methylphenidate studies that compared to a passive control showed statistically significant positive effects on broadband measures (SMD 0.66; 0.04, 1.28; 2 studies, n=302). The subgroup of alpha agonist studies reported a smaller effect, but the estimate was also statistically significant (SMD 0.45; CI 0.22, 0.68; 4 studies, n=509). The subgroup of NRI studies reported statistically significant effects and the effect size was between those of the stimulant and alpha agonist studies (SMD 0.53; CI 0.44, 0.63; 20 studies, n=3183).
For ADHD symptoms, the meta-regression suggested conflicting results with a statistically significant result for the overall test (omnibus test p 0.04) but not any of the individual parameters. The subgroup of amphetamine studies reported the largest and statistically significant effect (SMD −1.16; CI −1.64, −0.67; 5 studies, n=757). The subgroup of alpha agonist studies reported smaller but statistically significant effects (SMD 0.52; CI −0.67, −0.37; 11 studies, n=1885). The subgroup of NRI studies also reported statistically significant effects with the size of effect similar to alpha agonist studies (SMD 0.55; CI −0.62, −0.47; 28 studies, n=1925). The subgroup of methylphenidate studies reported slightly larger effects than the non-stimulant studies and the effect of the intervention was also statistically significant versus control (SMD −0.68; CI −0.91, −0.46; 7 studies, n=863).
Analyses for functional impairment did not detect a statistically significant difference in effect sizes (p 0.23). Insufficient studies were available for treatment satisfaction and academic performance.
For appetite suppression, the continuous outcome analysis did not detect a statistically significant effect (p 0.10), but the categorical outcomes indicated differences between intervention classes across studies (p 0.005). Specifically, the analysis suggested that amphetamine studies (p<0.001) and NRIs studies (p 0.02) report systematically different effect estimates from alpha agonist studies. The subgroup of amphetamine studies reported the largest and statistically significant effect (RR 7.08; CI 2.72, 18.42; 8 studies, n=1229). The subgroup of NRI studies reported smaller but also statistically significant effects (RR 3.23; CI 2.40, 4.24, 27 studies, n=4176). The subgroup of alpha agonist studies reported an even smaller effect (RR 1.49; CI 0.94, 2.37; 4 studies, n=919) and the difference to the control group was not statistically significant because the confidence interval just crossed the point of no effect. However, only guanfacine studies contributed to this finding and results for the drug class of alpha agonists are not known. The subgroup of methylphenidate studies reported statistically significant effects and effect sizes were between those of NRI studies and alpha agonist studies (RR 2.80; CI 1.47, 5.32; 8 studies, n=1110).
Analysis for the total number of participants reporting adverse events showed a borderline statistically significant effect (p 0.05), suggesting potentially differential effects for amphetamine studies (p 0.02) and NRI studies (p 0.03). The subgroup of amphetamine studies reported statistically significant effects (RR 1.41; CI 1.25, 1.58; 8 studies, n=1151). The subgroup of NRI studies reported a slightly smaller but statistically significant effect (RR 1.31; CI 1.18, 1.46; 15 studies, n=2600). The subgroup of alpha agonist studies reported a slightly smaller but also statistically significant effect (RR 1.21; CI 1.11, 1.31; 14 studies, n=2544). The subgroup of methylphenidate studies reported an effect most similar to NRI studies, and the estimate was also statistically significant (RR 1.32; CI 1.25, 1.40; 6 studies, n=945).
All analyses should be interpreted with caution as they are based on an indirect analysis across studies rather than on direct, head-to-head comparisons between medications.
5.3.2.3. FDA-Approved Pharmacologic ADHD Treatment Summary of Findings
Table 13 shows the findings for the outcomes of interest, together with the number of studies and study identifiers. We report the presence and absence of evidence for outcomes of interest, regardless of the number of identified studies. Effectiveness and adverse events analysis compared to control are shown first, followed by comparative effectiveness and safety analyses relative to an active comparator. For each outcome, results across all passive control groups are shown first, followed by specific comparisons (e.g., combinations vs individual components). In the comparative effectiveness section, we report first on the comparison between medication categories (stimulant vs non-stimulant), followed by the comparison between medication classes (amphetamines, methylphenidate, NRIs, alpha agonists). All other subgroup results for individual medications or medication classes are shown in this table only when we found empirical evidence of differences in effect sizes in direct or indirect comparisons. For any additional comparative effect analyses, such as comparisons between two medications (e.g., clonidine vs guanfacine), results are shown only when more than one study reported on the comparison (given that no studies or single studies would only add a row of insufficient evidence to the table).
The table states the comparison for which evidence is available, for example, we may have tried to determine the comparative effect of stimulants versus non-stimulants, but when all identified studies happened to test atomoxetine versus lisdexamfetamine (rather than the full range of non-stimulants and stimulants), we changed the comparison description to atomoxetine versus lisdexamfetamine.
Table 13
KQ2 summary of findings and strength of evidence for FDA-approved pharmacological interventions.
Across studies, we found high strength of evidence that ADHD medication had beneficial effects on broadband measures and ADHD symptom scores when comparing to passive control groups. We concluded high strength of evidence for broadband measure effects due to the consistency in direction of effects across studies, the large number of replications across independent author groups, the small amount of heterogeneity, the robustness of the finding when excluding high-risk of bias studies, and the absence of publication bias. Similarly, we concluded high strength of evidence for ADHD symptom measures due to the consistency in effects across studies, the large number of replications across independent author groups, the lack of substantial heterogeneity, the robustness of effects when excluding high-risk of bias studies, and the absence of publication bias. However, it should be noted that only few studies included children under six years of age in the evaluated interventions.
We downgraded the results for the subgroup of studies explicitly comparing the effect of non-stimulants plus stimulants to stimulants alone for inconsistency. We were unable to determine the effects across all studies as a study-level variable because identified studies varied in how rigorously they avoided co-interventions such as stimulant treatment; hence, it is unclear whether the documented subgroup is a good representation of all medication studies.
We also found moderate strength of evidence that pharmacological treatment reduces functional impairment, but we downgraded the strength of evidence due to observed heterogeneity.
Across studies, there was high strength of evidence that ADHD medication is associated with appetite suppression and that ADHD medication increases the risk of experiencing an adverse event compared to passive control groups. We concluded high strength of evidence for an increased risk due to the consistency of effects across studies, the large number of replications across independent author groups, the small amount of heterogeneity, the robustness of the finding when excluding high-risk of bias studies, and the robustness of the effect when using an alternative effect estimate that takes publication bias into account. We concluded high strength of evidence for an increased risk due to the consistency of effects across studies, the large number of replications by independent author groups, the small amount of heterogeneity, the robustness of the finding when excluding high-risk of bias studies, and the robustness of the effect when using an alternative effect estimate that takes publication bias into account.
The analyses comparing two alternative interventions and the corresponding strength of evidence were more limited. While NRIs had more favorable results than stimulants on problem behaviors, the number of studies and the effect was small, and the strength was downgraded due to study limitations. For the direct comparisons, we downgraded the strength of evidence for broadband measures and ADHD symptoms due to differences in direction of effects and study limitation. We downgraded the strength of evidence for appetite suppression for all comparisons due to differences in direction of effects, and some were further downgraded due to the small number of studies leading to imprecision All indirect comparisons were downgraded to low due to indirectness and imprecision where there were conflicting results between continuous and categorical variables.
5.3.3. Other Pharmaceutical Agents
We also identified studies evaluating a pharmaceutical agent not FDA-approved for ADHD.105, 113, 114, 122, 146, 147, 151, 155, 158, 165, 174, 206, 219, 232, 264, 269, 304, 354, 377, 399, 439, 507, 508, 513, 572, 574, 620, 636, 637 This included new formulations, off-label use of existing medication approved for other conditions such as modafinil, amantadine, or venlafaxine, and agents no longer available in the Unites States such as agomelatine. Identified studies were published between 1996 and 2022, with some only available as a trial record. Agents were evaluated in five different countries; with the majority of studies originating in the Unites States269, 377 and Iran.122, 219, 232, 354, 439, 508, 636 All studies used a randomized control trial design. Nearly all children within the studies received a confirmatory diagnosis by a specialist and/or clinician; exceptions507, 637 required only a preliminary clinical diagnosis. The populations were predominantly males between the ages of 6 and 18. Female population proportions ranged from 15 percent507 to 29 percent399 where reported. In nearly all studies, participants were required to demonstrate an IQ of 70 or higher. For studies that distinguished between ADHD presentations, the most prevalent (ranging from 58%507 to 100%354) was the combined presentation. Approximately half of studies did not report data regarding ADHD presentation type.113, 264, 269 The only study that addressed co-occurring disorders in the form of a dual diagnosis evaluated children with ADHD and mood disorders.377 Race and ethnicity demographics were described only in a portion of studies.113, 269, 377, 399
A variety of new pharmaceutical agents were tested for their efficacy in treating ADHD symptoms. Several studies evaluated the use of modafinil for youth with ADHD.122, 146, 147, 304, 354, 574 Modafinil is a stimulant medication that has been FDA-approved for the treatment of narcolepsy and sleep apnea. Two studies evaluated ABT-089, a neuronal nicotinic receptor partial agonist.105, 114 Two studies tested an inhibitor of G protein-coupled inward-rectifying potassium channels (GIRKs, tipepidine).219, 507 All of the studies evaluating pharmaceutical agents reported on a control group for some of the outcomes, which was typically placebo. The most common adjunctive treatment was methylphenidate. In addition to controls, several studies reported efficacy results for comparator groups, usually composed of participants who received a reduced dose of the pharmaceutical agent being tested. Studies reported a variety of study-specific outcomes, such as treatment-related adverse effects. In terms of pre-specified outcomes, broadband scale scores, standardized symptom scores, and appetite changes were the most frequently reported outcomes.
Only some of the identified studies reported sufficient detail to compute effect sizes for our key outcomes. The identified new agents are difficult to compare, particularly as they are chemically very diverse, and it is unclear whether any represent promising approaches for ADHD treatment. However, three agents were assessed in multiple studies.
5.3.3.1. Modafinil
The identified modafinil evaluation studies that reported on a broadband measure are shown in Figure 56.
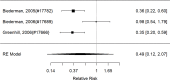
Figure 56
Effects of modafinil on broadband measures (RR). Notes: RE = random effects, RR = relative risk
Across studies, we did not detect a systematic effect of modafinil on broadband scores (RR 0.49; CI 0.12, 2.07; 3 studies, n=539). Two out of three studies were positive and there was heterogeneity (I-squared 76%). There was no indication of publication bias. None of the studies were considered high risk of bias, hence methodological rigor was not a likely source of heterogeneity.
Studies reporting on ADHD symptoms are shown in Figure 57.
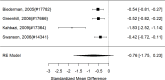
Figure 57
Effects of modafinil on ADHD symptoms (SMD). Notes: ADHD = attention deficit hyperactivity disorder, RE = random effects, SMD = standardized mean difference
Although all studies reported a positive effect, estimates varied and we did not find a statistically significant effect on ADHD symptoms due to wide confidence intervals (SMD −0.76; CI −1.75, 0.23; 4 studies, n=667). Heterogeneity was high (I-squared 91%). Results for publication bias were borderline (Begg p 1.00, Egger p 0.05) but the alternative estimate using the trim and fill method showed the same effect estimate. One study reported on the number of responders and found a large effect size given that most of the intervention participants showed at least a 40 percent decrease in the ADHD rating scores but none of the placebo participants did (RR 37.00; CI 2.36, 578.24; 1 study, n=46).354 Studies did not report on other outcomes other than appetite suppression (see Figure 58).
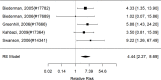
Figure 58
Effects of modafinil on appetite suppression (RR). Notes: ADHD = attention deficit hyperactivity disorder, RE = random effects, RR = relative risk
Modafinil significantly increased the risk of appetite suppression (RR 4.44; CI 2.27, 8.69; 5 studies; n=780). We detected no heterogeneity. We also found no indication of publication bias. None of the studies were categorized as high risk, hence it is unlikely that the result is purely based on methodological flaws of the studies.
5.3.3.2. Tipepidine
One study found no difference in a broadband measure (SMD 0.38; CI −0.17, 0.93; 1 study, n=51) or appetite suppression (RR 0.30; CI 0.01, 6.98; 1 study, n=51).219 Two studies reported on ADHD symptoms but estimates varied and no meaningful summary estimate could be derived (SMD −0.28, CI −3.59, 3.04; 2 studies, n=156).219, 507
5.3.3.3. ABT-089
Two studies by the same author group reported on α4β2 neuronal nicotinic receptor partial agonist for use in ADHD.105, 620 Both studies reported on a broadband measure but reported conflicting results and no meaningful summary measure could be derived (SMD −0.02, CI −2.58, 2.53; 2 studies, n=168). One of the studies reported on ADHD symptoms and found improvement (SMD −1.02; CI −1.46, −0.57; 1 study, n=88).624 Results for the number of participants reporting an adverse event are documented in Figure 59.
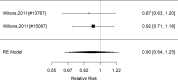
Figure 59
Effects of ABT-089 on participants reporting adverse events (RR). Notes: ABT-089 = a neuronal nicotinic receptor partial agonist, RE = random effects, RR = relative risk
Across studies, we found no statistically significant effect for an increased risk of adverse events (RR 0.90; CI 0.64, 1.25; 2 studies, n=171). We detected no heterogeneity, there was no effect of publication bias, and none of the studies were considered high risk.
5.3.3.4. Comparative Effects of Other Pharmacological Agents
We did not identify two studies comparing the same intervention and comparator. Some studies compared two different doses of the same agent.105, 146, 174, 269, 507, 513, 572 Multiple studies compared the evaluated intervention to methylphenidate,122, 165, 399, 439, 508, 636 and one study compared to atomoxetine.620 The others compared two different adjunctive treatments (risperidone vs divalproex)151 or different medication (risperidone vs aripiprazole).232 All individual studies are documented in detail in the evidence table in the appendix.
5.3.3.5. Summary of Findings, Other Pharmacological Agents
Given the diversity of agents that cannot be combined easily, no summary of findings across all studies could be established. Results of the individual studies are shown in Appendix C, Table C.2. The summary of findings table (Table 14) is limited to the agents assessed in multiple studies and Table 14 only shows results where effect size calculation was possible.
Table 14
KQ2 summary of findings and strength of evidence for other pharmacological agents.
Modafinil was associated with positive effects on ADHD symptoms (low strength of evidence, downgraded due to imprecision by 2). Modafinil was also associated with appetite suppression (moderate for effect). We did not find a positive effect on broadband measure scores, but the strength of evidence was limited (downgraded for study limitations).
The research benefit of ABT-089 is limited. We could not establish a meaningful effect estimate on broadband measures (downgraded to insufficient due to heterogeneity and imprecision). There was low strength of evidence (study limitation, imprecision) indicating that the intervention is associated with adverse events.
5.3.4. Youth-Directed Psychosocial Treatment
We identified 32 studies evaluating psychological, psychosocial, or behavioral interventions for children and adolescents with ADHD.106, 123, 160, 199, 204, 261, 290, 329, 330, 334, 335, 358, 392, 410, 426, 430, 471, 476, 480, 485, 521–523, 530, 532–535, 565, 594, 624, 643 We included studies in this section that evaluated psychosocial interventions targeting children or adolescents with ADHD, either alone or combined with components for the children’s parents or their teachers. The intervention category did not include combinations of psychosocial treatments plus medication; those were described in an earlier section. In addition, all interventions conducted in a school setting are documented in the school intervention section.
The earliest identified eligible study was published in 2003.123 Evaluations were conducted in 11 different countries, primarily the United States.106, 123, 204, 238, 261, 329, 476, 480, 522 The populations studied were children and adolescents with ADHD between the ages of “preschool” and 18, with half of the studies including teenagers. In studies that distinguished between ADHD presentations, the most prevalent type (ranging from 23.4%334 to 100%522 of the ADHD participants) was the combined presentation. While ADHD participants with co-occurring disorders were not excluded from most of the studies, three studies purposely included youth with language difficulties,624 homework problems,480 and organizational deficits.106 Race and ethnicity demographics were not mentioned in most studies.
Interventions studied were diverse and they differed in complexity and intensity. Intervention approaches included skills training (e.g., executive function training, homework, or organizational skills),106, 199, 204, 330, 426, 476, 480, 485, 521, 532–534 social skills training,123, 335, 392, 522, 565 executive function therapy for preschoolers,530 driving program for young drivers,261 sleep-focused intervention,329, 523 dialectical behavior therapy,430 cognitive behavior therapy,160 attention training,358 a complex behavior modification intervention,410 behavioral consultations with school and home components,204 parent-child training psychotherapy for mothers and their children who had ADHD,290 mindfulness training,535, 594 musicotherapy,643 play-based intervention,624 canine-assisted therapy,522 and one study compared a behavioral first strategy471 (providing a behavioral intervention before using medication). Many interventions had multiple components that involved patients, parents, teachers, therapists, and counselors in addition to direct interventions for the participating children. Interventions addressing parents exclusively are documented in the parent support section. Only half of the studies reported on a control group, including attention-matched groups or no intervention (i.e., wait list); the others compared to an alternative psychosocial treatment. Several compared against treatment as usual where it varied what treatment individual children received.
The most frequently reported outcomes in the included studies were the Conners Parent Rating Scales (CPRS), CGI scores, and the ADHD Rating Scale, Version IV.
Figure 60 shows the effect of the intervention on individual problem behaviors such as tardiness, delinquency, and conduct problems, assessed in the individual studies.
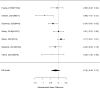
Figure 60
Effects of youth-directed psychosocial interventions on behavior (SMD). Notes: RE = random effects, SMD = standardized mean difference
Across studies, we did not detect a systematic effect of psychosocial interventions on problematic behaviors compared to control groups (SMD −0.18; CI −0.48, 0.12; 8 studies, n=947). The analysis did not detect substantial heterogeneity (I-squared 55%), but we note that one individual study unlike the other included studies reported a statistically significant effect. The training evaluated in the study focused on attention span, timetable activities, and homework compared to no intervention.358 We did not detect publication bias. Removing high-risk of bias studies in a sensitivity analysis left only two studies and showed a different estimate with wide confidence intervals, but the effect was still not statistically significant (SMD −0.12; CI −1.04, 0.80). One of the studies (evaluating a sleep-focused intervention) reported improvements in conduct problems after one year (SMD −0.34; CI −0.64, −0.05).523
One study reported on a broadband measure; the RCT found a statistically significantly positive effect (SMD 0.62; 0.24, 0.99; 1 study, n=120) for a multi-component, behavioral psychosocial treatment integrated across home and school (Child Life and Attention Skills) for youth with ADHD compared to families receiving a diagnostic report and a resource list.476
All studies reporting sufficient detail for changes on a continuous symptom scale are shown in Figure 61.
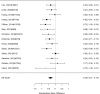
Figure 61
Effects of youth-directed psychosocial interventions on ADHD symptoms (SMD). Notes: ADHD = attention deficit hyperactivity disorder, RE = random effects, SMD = standardized mean difference
Analyses indicated a reduction in symptoms associated with a psychological or behavioral intervention (SMD −0.35; CI −0.51, −0.19; 14 studies, n=1686). Interventions were diverse and often included multiple components. Studies contributing to the results included a psychosocial intervention component directed at the children with ADHD; in some cases, however, an additional component addressed the parents or family specifically,204, 334, 335, 476, 485, 523, 532, 533, 535, 594 and some interventions involved the children’s teachers204, 476 in addition to the children and parents. Two studies evaluated STAND (Supporting Teens’ Academic Needs Daily), a parent-teen skills-based therapy blended with motivational interviewing that targets adolescents’ organization, time management, and planning occupational training skills, as well as parental monitoring and contingency management.532, 533 Particularly successful interventions included social skills plus parent skills training (compared to no intervention),335 a multi-component child life and attention skills program (compared to treatment as usual and a diagnostic report),476 ecological executive skills training with parent components (compared to waitlist),485 a family intervention focused on sleep (compared to usual care without focus on sleep management),523 family therapy STAND intervention (compared to usual care without family therapy),533 and a mindfulness training for children and parents (compared to waitlist).594 The youngest children included in the studies were 5 years old but several studies targeted pre-teens and teenagers. Statistical heterogeneity was not remarkable (I-squared 57%). There was no indication of publication bias. Most studies included in this analysis were RCTs; restricting to RCTs showed a similar effect estimate (SMD −0.36; CI −0.53, −0.19). Removing high-risk of bias studies in a sensitivity analysis left only seven studies but the effect estimate was similar (SMD −0.38; CI −0.69, −0.07).
One study reported on symptom improvement as a categorical variable; the study favored a multi-component, behavioral psychosocial treatment integrated across home and school (Child Life and Attention Skills) for youth with ADHD (RR 1.75; CI 1.14, 2.71; 1 study, n=114).476 Of all the psychosocial intervention studies, three reported long-term outcomes, which were statistically significant (SMD 0.52; CI 0.80, 0.23).334, 521, 523
Very few studies reported on functional outcomes. Two studies reporting on functional impairment as a categorical outcome could not be combined to a meaningful summary estimate (SMD 0.42; CI −1.13, 1.97; 2 studies, n=245).485, 523
Only one study reported sufficient detail to compute an effect size for treatment satisfaction, indicating no statistically significant difference between a parent-teen intervention focusing on safe driving and an attention-matched control group at the 12 month follow-up (SMD 0.19; CI −0.12, 0.49; 1 study; n=164).261
Studies reporting on academic outcomes and reporting sufficient detail to compute effect sizes are shown in Figure 62.
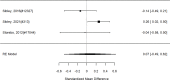
Figure 62
Effects of youth-directed psychosocial interventions on academic performance (SMD). Notes: RE = random effects, SMD = standardized mean difference
Across studies, we did not detect a systematic effect of the intervention on academic performance compared to control groups (SMD 0.07; CI −0.49, 0.62; 3 studies, n=459). The analysis detected little heterogeneity (I-squared 49%). There was no indication of publication bias. None of the studies included in this analysis was judged to be high risk of bias, suggesting that the lack of effect is not primarily driven by high-risk of bias studies.
Only one study formally reported on the number of participants with adverse events; the study found no increased risk associated with the social skills training intervention compared to treatment as usual as none of the groups reported any adverse events (RR 0.97; CI 0.02, 47.1; 1 study, n=55).565
5.3.4.1. Youth-Directed Psychosocial Treatment Comparative Effects
We identified a number of studies that compared diverse psychological and behavioral interventions to an alternative therapeutic approach.106, 160, 290, 330, 410, 430, 471, 476, 480, 521, 522, 534 None evaluated the same intervention, and comparators were also unique.
One study compared a group parent and adolescent skills training versus a dyadic skills training blended with motivational interviewing and reported similar results across assessed outcomes, including ADHD symptoms (SMD −0.23; CI −0.61, 0.16; 1 study, n=123).534 A study comparing two cognitive behavioral therapy programs (planning skills CBT versus solution-focused therapy CBT) reported initially more favorable results for the planning skills program, but the effect was not maintained, including for ADHD symptoms (SMD −0.14; CI −0.45, 0.17; 1 study, n=159).160 An evaluation of a problem-solving and organizational skills training for adolescents found no statistically significant difference in ADHD symptoms compared to progressive relaxation training (SMD −0.29; CI −0.74, 0.16; 1 study, n=77).521 Another study that focused on organizational functioning, time management, and planning in elementary school children found no statistically significant difference in a functional outcome (SMD 0.24; CI −0.11, 0.60; 1 study, n=125) or academic performance (SMD 0.13; −0.22, 0.48; 1 study, n=125) compared to a performance-based intervention that precluded skills training.106
One study in adolescents compared dialectical behavioral therapy compared to a psychoeducational group program about ADHD. It found lower self-reported ADHD ratings (SMD −0.39; CI −0.7, −0.08; 1 study, n=164) but no statistically significant difference for functional impairment (SMD 0.23; CI −0.08, 0.53; 1 study, n=164)430 Another of the identified studies evaluated a canine-assisted psychosocial intervention compared to behavioral parent training and social skills training.522 The study did not report sufficient detail to allow effect size calculations for the outcomes of interest but concluded that the canine-assisted group showed better results for ADHD symptoms.
A study comparing a multi-component program (Child Life and Attention Skills, CLAS) versus a parent-focused treatment with fewer school interactions, found the intensive program to have more positive effects, but there was no statistically significant difference in broadband measures (SMD 0.20; CI −0.13, 0.52 and RR 1.23; CI 0.89, 1.71; 1 study, n=199) or ADHD symptoms (SMD −0.27; CI −0.60, 0.05 and RR 1.23; CI 0.89, 1.71; 1 study, n=199).476 A family-school intervention versus an intervention about coping with ADHD through relationships and education (CARE) favored the family-school interventions for ADHD symptoms (SMD −0.34; CI −.061, −0.06; 1 study, n=199) but other outcomes assessed in the study did not show differences between interventions, including academic performance (SMD −0.21; −0.49, 0.07; 1 study, n=199).480 One study (n=145) compared a multi-component intervention of motivational components, homework management and schoolwork organization training, as well as family-school partnership building versus a complex medication integration protocol that included psychoeducation, medication decision-making, and integrated medication management. There were insufficient details reported to allow effect size calculations, but the authors concluded that both interventions showed positive effects.330 One study evaluating a complex intervention program consisting of parental training, behavior modification, sensory integration therapy, and sand tray therapy found no statistically significant difference compared to methylphenidate plus atomoxetine plus a homeopathic intervention for ADHD symptoms (SMD −0.35; CI −0.77, 0.07; 1 study, n=90).410
A study that included mothers with ADHD who had a child also diagnosed with ADHD evaluated parent-child training psychotherapy for mothers and children.290 The study found no statistically significant differences compared to individual non-specific counseling for the mothers for problem behaviors (SMD −0.10; CI −0.49, 0.30; 1 study, n=101), ADHD symptoms (SMD 0.19; CI −0.20, 0.59; 1 study, n=101), or functional impairment (SMD 0.11; CI −0.31, 0.52; 1 study, n=92) in the children with ADHD.
One study addressed sequencing of interventions.471 Children assigned to a multi-component behavioral intervention consisting of social skills training for children, parent training to establish a daily reward system, teacher consultations, and a case manager versus medication first reported significantly fewer classroom rule violations per hour than the medication first intervention (incidence rate ratio 0.66, p<0.01; 1 study, n=152). The study found no difference in the disruptive behavior disorder rating scales across groups (SMD −0.02; CI −0.34, 0.31; 1 study, n=152) or functional impairment (SMD −0.01; CI −0.33, 0.31; 1 study, n=152).
5.3.4.2. Youth-Directed Psychosocial Treatment Summary of Findings
Table 15 shows the findings for the outcomes of interest together with the number of studies and study identifiers. Findings are shown only when effect sizes could be computed.
Table 15
KQ2 summary of findings and strength of evidence for youth-directed psychosocial treatment.
The majority of psychological and behavioral interventions were multicomponent interventions and we found favorable effects of these on ADHD symptoms with a moderate strength of evidence. We downgraded all outcomes for study limitation as studies were at high or moderate risk of bias, often because studies of behavioral interventions versus no intervention cannot be blinded, and unblinded parents provided the outcome data. We found low strength of evidence that psychological interventions do not improve problem behaviors across studies and the evidence was insufficient for broadband measure scores. These findings were also downgraded for the domain inconsistency (direction of effects varied). There was insufficient evidence for functional outcomes due to additional imprecision as it was not clear whether or not psychological interventions influence functional impairment. Meta-analysis across studies found no difference in academic outcomes; strength of evidence is low due to inconsistency of direction and risk of bias. Only one study reported sufficient detail to compute effect sizes for treatment acceptability; the strength of evidence was rated insufficient. No studies reported on appetite changes or growth suppression, and only one study reported on the number of participants with adverse events; strength of evidence was determined to be insufficient, given the lack of data or inability to determine the consistency of effects where only one study reported on the outcome of interest.
The comparative effectiveness strength of evidence was determined to be insufficient due to the lack of studies reporting on similar interventions and comparators.
5.3.5. Cognitive Training
We identified 22 studies evaluating cognitive training to treat ADHD.56, 129, 139, 148, 166, 221, 222, 227, 229, 243, 258, 313, 367, 368, 372, 456, 457, 489, 578, 595, 613, 628 The earliest identified studies were from 2013.243, 578 Evaluations were published in 14 different countries, including the United States368, 372 and Iran.129, 456, 457
The populations studied were predominately males aged six to 17 years, with only one study including children as young as three years old.489 Evidence of intellectual disability (i.e., full-scale IQ < 70) was exclusionary in all studies, and eight studies required full-scale IQ scores of 80 or higher. Over 70 percent of studies included participants with a history of stimulant medication treatment, and of those, two thirds of their ADHD cohorts had prior or ongoing stimulant treatment. Five of the studies required stimulant treatment to be discontinued at least 24-hours before undergoing cognitive training, and several required an even longer washout period. For studies that distinguished between ADHD presentations (combined, inattentive, hyperactive/impulsive), the most prevalent was ADHD-combined type. While ADHD participants with typical co-occurring disorders such as conduct disorder were not excluded from most studies, a few studies purposefully included children with concomitant learning disorders (e.g., dyslexia, language disorder).222, 595 Race and ethnicity demographics were not mentioned in almost all studies.
Cognitive training interventions were delivered across different settings, including home-based and hospital/clinic-based programs. More than half of the studies used a computerized video game format such as the Cogmed digital working memory training program. Some studies used other non-computerized cognitive training modalities including structured, interactive games (e.g., Training Executive, Attention, and Motor Skills) and paper-and-pencil neuropsychological tasks, or they employed functional cognitive rehabilitation paradigms used in occupational therapy to improve ADHD as documented in detail in Appendix C, Table C.2. ADHD-matched control groups received treatment as usual,56, 166, 221, 367, 456, 613 or they were randomized to a waitlist or no intervention.139, 199, 243, 258, 313, 456, 578 Half the studies were compared to children exposed to non-adaptive/non-calibrated versions of the targeted cognitive intervention,148, 222, 229, 372 cognitive training of a separate domain (e.g., training of working memory vs. training of inhibitory control) or sham cognitive training227, 229, 368 or attention-matched intervention.129, 457 Other studies reported on the comparative effects for two alternative interventions without control group.368, 489, 595, 628
Studies reported a variety of study-specific outcomes, such as improvement in individual cognitive tasks. In terms of pre-specified key outcomes for this review, ADHD symptom rating scale scores were most frequently reported.
Studies that reported on a problem behavior are shown in Figure 63.
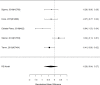
Figure 63
Effects of cognitive training on behavior (SMD). Notes: RE = random effects, SMD = standardized mean difference
Across identified studies, cognitive training had a positive effect on problem behaviors but the effect was only borderline statistically significant (SMD −0.43; CI −0.87, −0.00; 4 studies, n=267). This small set of studies did not detect heterogeneity or publication bias. All studies included in the analyses were RCTs. Removing two high-risk of bias RCTs resulted in a smaller estimate, but the effect was still statistically significant (SMD −0.26, CI −0.35, −0.18).
Studies reporting on broadband measure scores are documented in Figure 64.
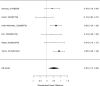
Figure 64
Effects of cognitive training on broadband measures (SMD). Notes: RE = random effects, SMD = standardized mean difference
The interventions were associated with a statistically significant improvement in broadband measures (SMD 0.52; CI −0.07, 0.96; 6 studies, n=344). Children included in the studies were between six and seven, and seven and ten, where reported. Heterogeneity was not remarkable (I-squared 58%) and there was no indication of publication bias. Removing high-risk of bias studies left only two studies with a smaller effect estimate that was no longer statistically significant due to wide confidence intervals (SMD 0.43; CI −0.54, 1.42). Similarly, restricting to parallel RCTs only found a smaller and not statistically significant effect (SMD 0.43; CI −0.06, 0.93). Only one study reported sufficient detail for a categorical analysis indicating no difference between groups (RR 0.96; CI 0.59, 1.55; 1 study, n=339).372
The studies reporting on the effect of cognitive training on ADHD symptoms are shown in Figure 65.
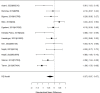
Figure 65
Effects of cognitive training on ADHD symptoms (SMD). Notes: ADHD = attention deficit hyperactivity disorder, RE = random effects, SMD = standardized mean difference
Across studies, we did not identify a systematic, statistically significant improvement of ADHD symptoms associated with cognitive training compared to control groups (SMD −0.36; CI −0.74, 0.01; 10 studies, n=499). The analysis did detect some heterogeneity (I-squared 69%). There was no evidence of publication bias. Removing four studies with high risk of bias also indicated a lack of systematic effect (SMD −0.24; CI −0.78, 0.30) and heterogeneity was not substantially reduced. An additional study reporting on a categorical symptom outcome (number with at least 30% improvement) did not detect statistically significant differences between groups (RR 1.28; CI 0.85, 1.94; 1 study, n=337).372
Studies reporting on effects of cognitive training on functional impairment are shown in Figure 66.
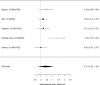
Figure 66
Effects of cognitive training on functional impairment (SMD). Notes: RE = random effects, SMD = standardized mean difference
Studies indicated an improvement in functional impairment, but the effect was not statistically significant (SMD 0.52; CI −0.34, 1.39; 4 studies, n=317). There was some heterogeneity and effect estimates varied somewhat (I-squared 79%). There was no indication of publication bias. Excluding two high-risk of bias studies in a sensitivity analysis (and thereby removing an outlier) did result in a smaller effect estimate that also was not statistically significant (SMD 0.27; CI −1.20, 1.74). An additional study reporting on impairment as a categorical variable did not detect differences between groups (RR 1.29; CI 1.00. 1.66, n=348).372
We could not compute effect estimates for treatment satisfaction in this intervention subset. Although two studies reported on an academic rating scale, estimates varied widely and we could not derive a meaningful summary estimate due to wide confidence intervals (SMD −0.72; CI ‑9.59, 8.15; 2 studies, n=68).129, 222
Appetite suppression was not assessed, but the number of participants experiencing an adverse event is shown in Figure 67.
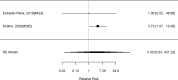
Figure 67
Effects of cognitive training on participants with adverse events (RR). Notes: RE = random effects, RR = relative risk
Only two studies reported clearly on the number of participants with adverse events in both treatment arms to determine the presence or absence of adverse events. Across studies, we did not detect a systematic effect of the intervention compared to a control group (RR 3.30; CI 0.03, 431.32; 2 studies, n=402). One of the studies reported no adverse events occurring in either study arm,258 the other reported more events in the intervention group, including frustration and headache (but no serious adverse events). In this small set of studies there was no evidence of heterogeneity and publication bias could not be assessed. Removing the high risk of bias study left one estimate that suggested a higher rate of adverse events in the intervention group (RR 3.73; CI 1.01, 10.83).372
5.3.5.1. Cognitive Training Comparative Effects
A small number of individual studies had active comparators. One study compared structured games versus parent training.489 The study did not report on key outcomes, but it concluded that working memory training is effective.
Four studies compared different cognitive training approaches.229, 368, 595 A study comparing central executive training versus inhibitory control training did not report on outcomes of interest in sufficient detail to allow us to compute effect sizes, but the study concluded that the finding supported the use of central executive training.368 Another study compared Cogmed working memory training versus a new active working memory and executive function compensatory training (paying attention in class).595 The study reporting finding no difference in a broadband measure, but it reported insufficient details to compute effect sizes. An additional study compared executive function training with multiple targets versus working memory training or inhibition and cognitive flexibility.229 The study did not report on key outcomes addressed in this review, but it concluded that there was no significant difference on any executive function measures. Another study compared two cognitive training batteries: ADHD executive functioning training versus general executive function training not specific to ADHD.628 The study reported no difference for ADHD symptoms (SMD 0.08; CI −0.33, 0.48; 1 study, n=94).
5.3.5.2. Cognitive Training Summary of Findings
Table 16 shows the findings for the outcomes of interest together with the number of studies and study identifiers. Comparative effectiveness and safety results are not shown as none of the identified studies reported on the key outcomes in sufficient detail.
Table 16
KQ2 summary of findings and strength of evidence for cognitive training.
Table 16 generally shows an emerging evidence base. Studies predominantly reported on specific measures rather than generally important outcomes such as ADHD symptoms. Strength of evidence was downgraded due to heterogeneity or inconsistency in direction of effects, and imprecision where no meaningful summary estimate could be derived from the available research. The evidence for multiple outcomes of interest is insufficient to date.
While different cognitive trainings have been compared in comparative effectiveness and safety evaluations, studies reported on study-specific intermediate outcomes, and it is unclear whether and which cognitive training is superior to others.
5.3.6. Neurofeedback
We identified 21 studies using neurofeedback.126, 130, 156, 215, 240, 280, 291, 294, 302, 320, 375, 398, 409, 435, 458, 483, 484, 490, 492, 562, 567 The earliest identified study was published in 2003.280 Studies came from 11 different countries, in particular Germany and the United States. Almost all studies used a randomized control trial study design, except for two non-randomized controlled studies,156, 302 The populations studied were between the ages of 6 and 18 years. Female population proportions in mixed samples ranged from 15398 to 37302 percent, and three studies did not include any girls.215, 484, 492 In nearly all studies, participants were required to demonstrate an IQ of 80 or higher. For studies that distinguished between ADHD presentations, the most prevalent type, ranging from 15492 to 100567 percent of ADHD participants, was the combined type. There were no reported systemic co-occurring disorders within the included study populations, though many did not exclude commonly associated co-occurring disorders within their study population. Race and ethnicity demographics were described in few of the identified studies.458, 562
A variety of neurofeedback protocols were tested for their efficacy in treating ADHD symptoms. Two thirds involved theta/beta electroencephalogram (EEG) marker modulation.126, 130, 156, 172, 215, 240, 291, 294, 302, 398, 458, 492, 562 One third of protocols centered around modulation of slow cortical potentials.294, 320, 375, 435, 567 Among the neurofeedback studies, three quarters reported on a passive control group, including attention-matched task,215, 291 waitlisted for intervention,398, 492 and no intervention groups.302, 562 Several studies reported efficacy results compared to an alternative intervention, most frequently cognitive training or methylphenidate.
Studies reported a variety of often study-specific outcomes, such as improvement in individual cognitive tasks as documented in Appendix C, Table C.2. In terms of pre-specified outcomes, broadband scale scores and standardized symptom scores were the most frequently reported outcomes.
Studies reporting on reductions in problematic behaviors, such as aggression and off-task behavior at school, are shown in Figure 68.

Figure 68
Effects of neurofeedback on behavior (SMD). Notes: RE = random effects, SMD = standardized mean difference
Study results varied considerably, and no systematic effect was seen across studies (SMD ‑0.33; CI −1.33, 0.66; 3 studies, n=372). Despite the small number of studies, the analysis detected heterogeneity (I-squared 86%). There was no indication of publication bias, and removing a high-risk study did also not indicate a statistically significant effect (SMD −0.52; CI −2.00, 0.97). Two of these studies reported long-term behavior improvements, but estimates varied, and no meaningful summary estimate could be derived (SMD −0.21; CI −1.55; 1.12).126, 458
Two studies reported on a continuous broadband measure as shown in Figure 69.

Figure 69
Effects of neurofeedback on broadband measures (SMD). Notes: RE = random effects, SMD = standardized mean difference
Although studies reported positive effects, the summary estimate was not statistically significant (SMD 0.42; CI −0.08, 0.93; 2 studies; n=283). Heterogeneity was not detected, and there were too few studies for further analyses. Of these, one reported significant improvement126 after 25 months (SMD 0.38; CI 0.01, 0.74).126 The equivalent analysis for a categorical outcome is shown in Figure 70.
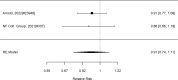
Figure 70
Effects of neurofeedback on broadband measures (RR). Notes: RE = random effects, SMD = standardized mean difference
Although studies reported positive effects, the individual nor the pooled studies were not statistically significant (RR 0.91; CI 0.74, 1.11; 2 studies, n=262). Both studies reported long-term outcome effects.
Results for ADHD symptoms are reported in Figure 71.
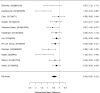
Figure 71
Effects of neurofeedback on ADHD symptoms (SMD). Notes: ADHD = attention deficit hyperactivity disorder, RE = random effects, SMD = standardized mean difference
Across studies, neurofeedback was associated with a statistically significant ADHD symptom reduction compared to different passive control groups (SMD −0.47; CI −0.72, −0.22; 11 studies, n=857). The youngest children included in the studies were 6 years old. The analysis detected little heterogeneity (I-squared 54%). Excluding six high-risk of bias studies (i.e., half of all included studies) resulted in a similar effect estimate but also wider confidence intervals and consequently, the effect was no longer statistically significant (SMD −0.41; CI −0.78, −0.05). Similarly, restricting to sham-controlled studies only resulted in the same effect estimate, but due to larger confidence intervals, the effect was not statistically significant (SMD −0.42; CI −1.31, 0.48). This group includes controlled trials without random assignments; restricting to the nine RCTs found the same point estimate as the overall analysis and the result remained statistically significant (SMD −0.47; CI −0.79, −0.15). Analyses also suggested the presence of publication bias (Begg p 0.01, Egger p 0.01). However, the trim and fill method did not suggest a different effect estimate (SMD −0.43; CI −0.68, −0.18). One of the included studies reported a statistically significant long-term effect (SMD 0.35; CI 0.68, 0.010) for a continuous outcome,458 but a second study reporting categorical improvement did not (RR 0.91; CI 0.72, 1.14).126
Studies reporting on functional impairment outcomes are shown in Figure 72.
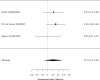
Figure 72
Effects of neurofeedback on functional impairment (SMD). Notes: RE = random effects, SMD = standardized mean difference
Studies did not indicate a systematic effect of neurofeedback on functional impairment (SMD 0.21; CI −0.14, 0.55; 3 studies; n=332). Statistical heterogeneity was limited (I-squared 49%). Two of the studies reported long-term improvement, but the effect was not statistically significant (SMD 0.26; CI −0.24, 0.76).126, 458
We did not identify treatment satisfaction or academic performance estimates. One study reported on appetite suppression and found no systematic difference between intervention and control groups (RR 1.45; CI 0.68, 3.10; 1 study, n=142).458 Identified studies did not report on the number of participants with adverse events.
5.3.6.1. Neurofeedback Comparative Effects
Seven studies reported on active comparators, including cognitive training,294, 320, 435, 562 medication with methylphenidate,291, 483 and electromyographic biofeedback,215 as documented in the next subsections.
5.3.6.1.1. Neurofeedback Versus Cognitive Training
Two studies reported on individual behaviors as documented in Figure 73.
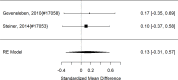
Figure 73
Neurofeedback versus cognitive training on behaviors (SMD). Notes: RE = random effects, SMD = standardized mean difference
Across studies, we found no statistically significant difference between neurofeedback and cognitive training, but the number of identified studies contributing to the comparison was small (SMD 0.13; CI −0.31, 0.57; 2 studies, n=129). The set did not identify heterogeneity; both studies were classified as high risk of bias.
The identified studies did not compare the effect of neurofeedback and cognitive training on broadband measures.
Results for ADHD symptoms are shown in Figure 74.
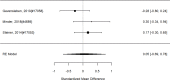
Figure 74
Neurofeedback versus cognitive training on ADHD symptoms (SMD). Notes: ADHD = attention deficit hyperactivity disorder, RE = random effects, SMD = standardized mean difference
Across studies, we found no systematic difference between interventions (SMD 0.05; CI −0.69, 0.78; 3 studies, n=167) and little heterogeneity was detected (I-squared 15%) in this small set of studies (all judged to be high risk of bias). One study reported on a categorical outcome (number of responders) and also found no statistically significant difference (RR 1.34; CI 0.76, 2.37; 1 study; n=77).435
Two studies reported on a functional impairment measure. Both reported no statistically significant difference between interventions, but estimates varied, and the studies could not be combined to a meaningful effect estimate (SMD 0.10; CI −1.35, 1.56; 2 studies, n=133) given the wide confidence intervals.294, 562 We did not identify studies that evaluated neurofeedback versus cognitive training that reported on other outcomes of interest for the review.
5.3.6.1.2. Neurofeedback Versus Stimulants
Two studies were identified that made comparisons to medication, and each one reported on some of the outcomes of interest. One study compared personalized at-home neurofeedback training versus methylphenidate.483 The study found more improvement in broadband measures in the medication group compared to neurofeedback (RR 3.61; 2.36, 5.52; 1 study, n=149).
Both studies reported on ADHD symptom measures comparing neurofeedback versus methylphenidate.291, 483 Both studies found more improvement associated with methylphenidate, but effect estimates differed and resulted in wide confidence intervals, precluding a meaningful effect estimate (SMD 0.52; CI −1.29, 2.34; 2 studies, n=209).291, 483
One of the studies reported adverse events; it found significantly fewer participants experienced adverse events in the neurofeedback versus the methylphenidate group (RR 0.23; CI 0.15, 0.35; 1 study, n=149).483
5.3.6.2. Neurofeedback Summary of Findings
Table 17 shows the findings for the outcomes of interest, together with the number of studies and study identifiers for the key outcomes. Comparative effects are shown when more than one study was identified that reported on the outcome.
Table 17
KQ2 summary of findings and strength of evidence for neurofeedback.
The summary of findings table (Table 17) shows an improvement for ADHD symptom scores compared to passive control (low strength of evidence, downgraded for study limitation due to the large number of high-risk of bias studies and inconsistency in effect estimates). Results for other outcomes were less favorable or unclear. For all outcomes, we downgraded for imprecision where no summary estimate could be derived. We downgraded the strength of evidence for appetite suppression due to lack of replication (only one study reported on this outcome of interest). It should be noted that the included neurofeedback approaches varied by study, and results reported in the individual studies are shown in the evidence table in more detail.
We detected no systematic difference between neurofeedback and cognitive training in the small number of studies that reported on this comparison for the outcomes of interest. We upgraded the evidence for broadband measure scores comparing neurofeedback versus methylphenidate due to the large effect. All other comparisons were downgraded for the domain inconsistency by two (results were based on a single study, and it was not possible to determine whether another study by another author group would report an effect) and study limitation (unclear whether the study was statistically powered to detect an effect for the outcome).
5.3.7. Neurostimulation
We identified one study evaluating neurostimulation that met eligibility criteria.517 The study was an RCT conducted in Israel. The proportion of girls was 28 percent. It included youth with inattentive, hyperactive, and combined ADHD presentation. The study evaluated a transcranial direct current stimulation protocol plus cognitive therapy compared to sham neurostimulation plus cognitive therapy.
The study did not find an effect on the CBCL (Child Behavior Checklist) total score (SMD 0.19; CI −0.60, 0.97, 1 study, n=25). There was also no statistically significant improvement in ADHD symptoms based on the Vanderbilt scale score (SMD −0.58; CI −1.39, 0.22; 1 study, n=25). The study did not report on any other outcomes of interest that allowed calculation of an effect size, but it noted that three children in the active stimulation group reported headaches resulting in withdrawal of one child and temporary suspension of the intervention for the other two children.517
The summary of findings table (Table 18) summarizes the findings across studies.
Table 18
KQ2 summary of findings and strength of evidence for neurostimulation.
We downgraded all outcomes to insufficient. Although the one identified study reported on a broadband measure and ADHD symptoms, the study was small and likely not powered for the documented effect size calculation.
5.3.8. Physical Exercise
We identified seven studies reporting on physical exercise interventions that met eligibility criteria.180, 239, 345, 353, 396, 406, 503 Studies were conducted in China, Germany and Switzerland, Korea, Taiwan, Tunisia, and Turkey. None of the studies were conducted in the U.S. The percent of female participants ranged from 10406 to 23,396 where reported.
Studies addressed very different interventions. Two studies evaluated a martial arts intervention.353, 406 One study each reported on the effects of treadmill training plus whole body vibration,239 table tennis training,180 aerobic and neurocognitive exercise,396 physiotherapeutic treatment,503 and exergaming using a running or jumping board with connected screen.345
With one exception, the identified studies did not report on the prespecified outcomes, nor did they report on the outcomes with sufficient detail to compute effect sizes. One RCT published in 2020239 compared treadmill training plus whole body vibration training, versus treadmill training alone, in children with ADHD. The study was conducted in Turkey; children ranged in age from seven to 11 years and were treatment naïve. Eighty percent of participants had combined type ADHD and the same percentage were male. The study reported no difference between groups (SMD 0.20; −0.51, 0.92; 1 study, n=30) for a broadband measure. Other results are shown in the evidence table in the appendix.
5.3.8.1. Exercise Comparative Effectiveness
Two of the identified studies had an active comparison group. A study evaluating physiotherapeutic treatment to train motor skills versus methylphenidate did not report sufficient detail to allow effect size calculation for any of the outcomes of interest, but the study concluded that there is no clear evidence for beneficial effects of methylphenidate or physiotherapeutic treatment on children’s overall graphomotor movements.503 A study evaluating the therapeutic effect of table tennis training compared to simulated table tennis did not also not report sufficient detail for effect size calculations; the study concluded that table tennis motor coordination activities improve executive functions and handwriting problems.
5.3.8.2. Exercise Summary of Findings
Table 19 below shows the effect estimates for the outcomes of interest.
Table 19
KQ2 summary of findings and strength of evidence for physical exercise.
Given the lack of studies or lack of replication of effects in more than one study, we determined evidence for all outcomes of interest to be insufficient.
5.3.9. Nutrition and Supplements
We identified 39 studies of nutrition or supplement interventions. The vast majority were placebo-controlled studies of dietary supplements. Several evaluated nutritional supplements as augmentation to stimulant medication. The earliest eligible study was published in 2004. Only two of the identified studies were conducted in the United States.350, 606 Most others were conducted in the Middle East or Europe and only three were conducted in the United States.350, 601, 606 All studies but one (which included children as young as four)472 enrolled children at least six years of age. Race and ethnicity were rarely reported, perhaps due to the racial homogeneity of the trial locations. Two studies had no females,209, 364 while the others reported including between six and 45 percent included girls. ADHD presentations were rarely reported. Children with psychological and psychiatric co-occurring disorders were excluded from at least half the studies. One studied children with co-occurring epilepsy,262 one study included children with chronic sleep-onset insomnia.596 and one478 included children with iron deficiency.
The studies assessed a wide range of dietary and supplement approaches. Omega 3 fatty acid (DHA and/or EPA) was evaluated in 13 studies.138, 171, 178, 209, 212, 262, 310, 318, 349, 411, 441, 510, 601 Three studies evaluated vitamins.324, 350, 505 Two studies evaluated saffron136, 363 two evaluated zinc sulfate,116, 149 The DASH (Dietary Approaches to Stop Hypertension) diet,364 an individually designed restricted elimination diet,472 and a further dietary intervention296 were also studied. Two studies evaluate melatonin.440, 596 The most common categories of outcomes were broadband and ADHD symptom scores. In terms of instruments, CPRS and the ADHD Rating Scale, 4th Version (ADHD RS-IV) were the most frequently reported outcome measures.
Figure 75 shows results for individual problem behavior such as teacher-reported conduct problems evaluated in individual studies; the figure is ordered by dietary supplement.
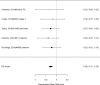
Figure 75
Effects of nutrition or supplements on behavior (SMD). Notes: PS = phosphatidylserine, RE = random effects, SMD = standardized mean difference
Across studies, nutritional approaches (docosahexaenoic acid, phosphatidylserine, vitamins and minerals, sarcosine), were associated with improvement in problem behavior compared to control (SMD −0.28; CI −0.37, −0.18; 5 studies, n=360). None of the studies included children under six years of age. There was no evidence of heterogeneity and publication bias was not detected. All studies used random assignment to treatment groups and excluding one high risk of bias study found a similar effect (SMD −0.25; CI −0.33, −0.17). The included omega 3 study (n=49), the most commonly evaluated nutrition or supplement intervention in this subgroup, reported no statistically significant differences, and heterogeneity could not be determined (SMD 0.18; CI −0.38, 0.75).212
Results of nutrition and supplements on broadband measures are shown in Figure 76.
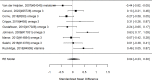
Figure 76
Effects of nutrition or supplements on broadband measures (SMD). Notes: RE = random effects, SMD = standardized mean difference
Across studies, we did not detect a consistent effect of the intervention compared to control (SMD 0.04; CI −0.23, 0.30; 9 studies, n=953). There was some evidence of heterogeneity (I-squared 66%). Heterogeneity was not explained by risk of bias; excluding two high-risk of bias studies resulted in a very similar estimate (SMD 0.06; CI −0.31, 0.44) and heterogeneity increased. There was no evidence of publication bias. The most common supplement assessed in this category was omega 3 and when restricting to omega 3 studies, results for broadband measures were similar in not showing a systematic benefit across seven studies (n=755) and there was less heterogeneity (SMD 0.04; CI −0.24, 0.32; I-squared 54%).171, 209, 212, 310, 349, 411, 510 A few studies assessed the number of participants that improved (categorical measure) according to a broadband measure as shown in Figure 77.

Figure 77
Effects of nutrition or supplements on broadband measures (RR). Notes: ALC = acetyl-L-carnitine, RE = random effects, RR = relative risk
Similar effects are shown for broadband measures used as a categorical variable and the analysis did not detect a systematic treatment effect (RR 0.68; CI 0.32, 1.43; 4 studies, n=385). The studies assessed different interventions, including a metabolite for energy metabolism,125 micronutrients,350 vitamin-mineral treatment,505 and St. John’s Wort606 and there was some evidence of heterogeneity (I-squared 73%). None of the studies were judged to be high risk of bias. There no indication of publication bias.
All studies reporting on the effects of nutrition or supplements on ADHD symptoms are shown in Figure 78.
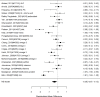
Figure 78
Effects of nutrition or supplements on ADHD symptoms (SMD). Notes: ALC = acetyl-L-carnitine, PS = phosphatidylserine, RE = random effects, SMD = standardized mean difference
Across studies, analyses for the nutritional approaches and supplements showed a positive effect on ADHD symptoms compared to control (SMD −0.39; CI −0.67, −0.12; 23 studies, n=2357). The youngest children included in the studies were four years old. There was considerable heterogeneity (I-squared 89%) in results across studies. The largest effects were reported by a study evaluating a zinc sulfate supplement149 and a restricted elimination diet.472 There was no evidence of publication bias. Most identified studies were RCTs; restricting to parallel RCTs exclusively found a similar effect (SMD −0.32; CI −0.55, −0.08). Excluding four high-risk of bias studies suggested a smaller treatment estimate but the result was still statistically significant (SMD −0.26; CI −0.52, −0.01), and heterogeneity was not reduced. An omega 3 supplement was the only comparable intervention that was studied in more than one of the otherwise very diverse studies. Restricting to the seven omega 3 studies (n=719) did not find any benefits of the supplement (SMD −0.11; CI −0.45, 0.24; I-squared 71%).171, 178, 209, 212, 318, 349, 441 The studies reporting on symptom improvement as a categorical variable (i.e., number of participants showing a treatment response) are shown in Figure 79.
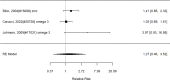
Figure 79
Effects of nutrition or supplements on ADHD symptoms (RR). Notes: RE = random effects, RR = relative risk
Studies did not indicate a statistically significant effect of nutrition interventions on ADHD symptoms when using a categorical outcome. (RR 1.27; CI 0.46, 3.52; 3 studies, n=416). Despite the small number of studies, some heterogeneity was detected (I-squared 24%). There was no evidence of publication bias. Two studies (n=224) with a categorical ADHD symptom measure evaluated omega 3; the studies found no statistically significant effect (RR 1.67; 0.00, 6502; I-squared 68%),171, 349 heterogeneity was not reduced, and the estimate was very imprecise.
Effects of nutrition and supplements on functional outcomes are shown in Figure 80.
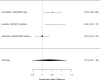
Figure 80
Effects of nutrition or supplements on functional impairment (SMD). Notes: RE = random effects, SMD = standardized mean difference
Across available studies reporting sufficient detail for effect size calculations, no systematic benefit was found for functional impairment (SMD 0.37; CI −0.52, 1.26; 3 studies, n=272). Studies evaluated different interventions, including vitamin D plus magnesium,324 micronutrients,350 and the DASH diet.364 Despite the small number of studies, the analysis detected heterogeneity (I-squared 65%).
There were no data for treatment acceptability or academic performance.
A few studies assessed continuous variables indicative of appetite suppression, such as height, body mass index (BMI), and weight changes as shown in Figure 81.
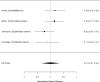
Figure 81
Effects of nutrition or supplements on appetite suppression (SMD). Notes: ALC = acetyl-L-carnitine, RE = random effects, SMD = standardized mean difference
There were no differences between treatment arms (SMD 0.01; CI −0.31, 0.33; 4 studies, n=485) for appetite suppression measures. Heterogeneity was negligible (I-squared 19%). There was no indication of publication bias. Removing one high risk of bias study showed no effect either (SMD −0.05; CI −0.58, 0.48). One of the studies assessed omega 3 specifically (n=162); the study did not detect a statistically significant effect (SMD 0.16; CI −0.17, 0.49; I-squared 0).411 The equivalent analysis for a categorical outcome (number of participants reporting appetite suppression) is shown in Figure 82.

Figure 82
Effects of nutrition or supplements on appetite suppression (RR). Notes: ALC = acetyl-L-carnitine, PS = phosphatidylserine, RE = random effects, RR = relative risk
The equivalent analyses for a categorical outcome came to similar conclusions and did not detect an effect on appetite suppression (RR 1.10; CI 0.88, 1.38; 6 studies, n=439). The analysis did not detect heterogeneity. There was some indication of publication bias (Begg p 0.06, Egger p 0.02). An alternative estimate using the trim and fill method also showed no systematic benefit (RR 1.16; CI 0.89, 1.51). Removing a high-risk of bias study in a sensitivity analysis found a similar effect (RR 1.14; CI 0.88, 1.48) suggesting that the result was not primarily driven by poor methodology.
Studies evaluating the effects of nutrition or supplements on adverse events are shown in Figure 83.

Figure 83
Effects of nutrition or supplements on participants with adverse events (RR). Notes: RE = random effects, RR = relative risk
Across studies, there was no indication that the interventions were associated with a higher risk of experiencing an adverse event (RR 0.77; CI 0.47, 1.27; 8 studies, n=735). Heterogeneity was negligible (I-squared 26%), there was no evidence of publication bias, and none of the studies contributing to the effect estimate were considered high risk of bias. This analysis included four omega 3 studies. The result for this subset (n=398) was similar to the overall analysis and omega 3 was also not associated with an increased risk of experiencing adverse events (RR 0.87; CI 0.48, 1.56; I-squared 0).
5.3.9.1. Nutrition and Supplements Comparative Effects
Few of the nutrition and supplement studies used active comparators comparing the nutrition or supplement to a different intervention.
Three studies compared to methylphenidate while the intervention group received saffron,136 sweet almond syrup,444 or ginkgo biloba509 Two of the studies reported on symptoms but they found conflicting results. One reported no difference between saffron versus methylphenidate groups, while one favored methylphenidate over ginkgo biloba and the studies could not be combined to a meaningful summary estimate (SMD 0.40; CI −4.80, 5.59; 2 studies, n=100). However, both studies reported also on the outcome appetite suppression as shown in Figure 84.
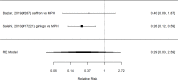
Figure 84
Nutrition or supplements versus methylphenidate on appetite suppression (RR). Notes: RE = random effects, RR = relative risk
Both studies found more events in the methylphenidate groups but due to the small number of studies and differences in effect sizes, the pooled effect was not statistically significant (RR 0.29; CI 0.03, 2.59; 2 studies, n=100).
One study compared omega 3 versus zinc supplements and found no difference in a broadband measure (SMD 0.02; CI −0.37, 0.41; 1 study, n=150).510
5.3.9.2. Nutrition and Supplements Summary of Findings
Table 20 displays the findings for each outcome category along with the number of studies and study identifiers. The summary of findings table displays data for all outcomes of interest across all nutrition/supplements. In addition, the table shows the effects for specific supplements where more than one study reported on the particular agent for the outcome; only Omega 3 was evaluated in more than one study reporting on the same outcome. Results of the individual studies are documented in Appendix C, Table C.2.
Table 20
KQ2 summary of findings and strength of evidence for nutrition and supplements.
The majority of studies reported on ADHD symptoms, and we found low strength of evidence that nutrition and supplements can show benefits. We downgraded by two for inconsistency since we only found effects for one outcome type (continuous, not categorical data) and the continuous data showed considerable heterogeneity. In addition, the evaluated supplements and dietary approaches were very diverse. And it was not possible to identify an effect of a specific intervention that has shown positive effects in more than one study. There was also a positive effect shown for individual problem behaviors, but the number of studies and samples were small, none of the individual studies reported statistically significant effects, and an additional study may change the statistical significance of the pooled effect (downgraded by two for imprecision). We found no effect on broadband measures and no statistically significant difference between study arms for functional impairment; we downgraded the strength of evidence due to heterogeneity (inconsistency). There was insufficient evidence to estimate the effect on acceptability of treatment and academic performance due to the lack of research studies. There was moderate strength evidence that nutrition and supplement interventions are just as safe as a placebo, but we downgraded for study limitation as some studies had reported adverse events but did not report on the number of participants experiencing adverse events.
The evaluated supplements and dietary approaches were very diverse but the effect of omega 3 has been assessed in multiple studies. We found no evidence that omega 3 improves behavior, broadband measure scores, or ADHD symptoms, and it was not associated with appetite suppression or experiencing adverse events. We downgraded the omega 3 evidence due to study limitations.
We found two studies that reported the comparative effectiveness of supplements versus methylphenidate. While both reported on ADHD symptoms, we determined the strength of evidence to be insufficient because of the small number of studies reporting on two different supplements (inconsistency), studies reported conflicting results (inconsistency) and no meaningful summary estimate could be derived (imprecision). There was low strength of evidence that supplements reported fewer appetite suppression events than methylphenidate (downgraded for inconsistency and imprecision). We downgraded the strength of evidence for no difference between omega 3 and zinc in broadband measures to insufficient (study limitation, downgraded by two as the single study did not let us assess inconsistency).
5.3.10. Complementary, Alternative, or Integrative Medicine
We identified six studies that evaluated complementary, alternative, or integrative medicine interventions.128, 150, 278, 279, 332, 646 Studies were published between 2001 and 2022; they were conducted in Switzerland,278, 279 China,646 Iran,150 Israel,128 and Korea.332 All studies included both children and adolescents and participants were predominately male. Race or ethnicity of the included study participants was not reported. ADHD presentations were also not reported. Studies evaluated acupuncture, homeopathy, and hippotherapy. Three studies compared to a passive control group (waitlist, placebo, attention-matched control).
None of the studies reported on individual problem behaviors.
Two of the identified studies reported on a broadband measure in sufficient detail to calculate an effect size, but the estimates varied greatly, and no meaningful summary estimate could be derived (SMD 0.03; CI −3.66, 3.73; 2 studies, n=218).332, 646 One acupoint stimulation study reported a positive effect on a categorical broadband measure (RR 0.23; CI 0.07, 0.75; 1 study, n=78).646
The studies reporting on ADHD symptoms are shown in Figure 85.
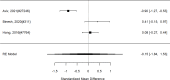
Figure 85
Effects of complementary, alternative, or integrative medicine on ADHD symptoms (SMD). Notes: ADHD = attention deficit hyperactivity disorder, RE = random effects, SMD = standardized mean difference
We did not detect a systematic effect of interventions (SMD −0.15; CI −1.84, 1.53; 3 studies, n=313). The studies evaluated hippotherapy, traditional acupuncture, and auricular acupuncture. The positive effect was reported by a study evaluating therapeutic horseback riding.128 One of the studies reported on symptom improvement as a categorical variable and found auricular acupuncture improved symptoms (RR 4.26; CI 1.42, 12.77; 1 study, n=44).150
None of the identified studies reported sufficient detail to calculate effect estimates for the other outcomes of interest, including functional impairment, treatment satisfaction, academic performance, and appetite suppression.
One study evaluating transcutaneous electrical acupoint stimulation reported on the number of participants with adverse events. The study did not demonstrate a statistically significant effect of the intervention compared to sham treatment (RR 2.00; CI 0.19, 21.16; 1 study, n=78) and it reported that adverse events were rare and not serious.646
5.3.10.1. Complementary, Alternative, or Integrative Medicine Comparative Effects
One of the identified studies (n=115) compared homeopathy and methylphenidate.279 The high risk of bias study used the CGI scale but did not provide sufficient detail to allow computation of effect sizes. The authors concluded that homeopathic treatment appears to be similar to the effect of methylphenidate.
5.3.10.2. Complementary, Alternative, or Integrative Medicine Summary of Findings
Table 21 shows the findings for the outcomes of interest together with the number of studies and study identifiers.
Table 21
KQ2 summary of findings and strength of evidence for CAM.
Very few studies reported on the key outcomes selected for the review and the conclusion for the outcomes was that the evidence base is insufficient because of lack of research, conflicting results, and lack of replication of effects for specific integrative or alternative medicine approaches. The strength of evidence was determined to be insufficient for broadband measure scores due to inconsistency and imprecision. Studies evaluated different interventions, and no meaningful summary estimate of the intervention group could be derived. The strength of evidence was determined to be insufficient for symptoms because of conflicting results across studies and lack of meaningful summary estimate; it is unclear whether complementary, alternative, or integrative medicine interventions have an effect on ADHD symptoms. Similarly, the strength of evidence was determined to be insufficient for the number of participants with adverse events. Given the variation in approaches, the identified study is unlikely a good representation of expected adverse events for this intervention group, and only one of the identified studies reported on the outcome.
Only one comparative effectiveness study was identified, and it reported insufficient details to compute effect sizes for the outcomes of interest; hence the strength of evidence was determined to be insufficient.
5.3.11. Parent Support
We identified 19 studies evaluating an intervention primarily targeting parents.110, 176, 200, 228, 257, 265, 266, 325, 333, 384, 428, 520, 544, 550–552, 569, 585, 593 Some psychosocial studies presented earlier in the chapter also included a parent component, but as an addition to targeting the children and adolescents directly. The studies in this section do not mention a component directed at the youth with ADHD and instead focus on the parents. The earliest identified parent support study was published in 2001.550 Evaluations were published in 13 different countries, primarily the United States110, 176, 200, 325 and the UK.266, 550–552 The populations studied were parents of children with ADHD between the ages of three and up to 18 years, but only three studies reported on parents of teenagers with ADHD.200, 265, 266 For studies that distinguished between ADHD presentations, the most prevalent type of the ADHD participants was the combined type. While ADHD participants with co-occurring psychiatric disorders were not excluded from most of the studies, only one study purposely included specific co-occurring disorders; the study included youth with a dual diagnosis of ADHD and oppositional defiant disorder.257 One study included children with sleep problems428 Race and ethnicity demographics for the parents or children were not mentioned in most studies.
Interventions were diverse in terms of the approach as well as intensity and included behavioral training for parents, in-home nurse visits, group psychotherapy, telephone-assisted self help, psychoeducation, and parental friendship coaching. One intervention each targeted sleep or reading, several evaluated the New Forest Parenting Program. Of the identified studies, most reported on a control group, including attention-matched groups,265, 290 no intervention, waitlist, or treatment as usual.228, 266, 329, 384, 520, 551, 585 Some studies included both a control group and an alternative psychological or behavioral intervention, had only an alternative intervention as comparison, or compared parent training as stimulant augmentation to medication alone.
Although we did not restrict the type, target, or focus of the intervention (i.e., either primarily addressing the wellbeing of parents or training parents to affect change in the children with ADHD), we only included studies that reported data on the effects on the children with ADHD; studies reporting only on parental outcomes were excluded (see Table 1). Studies reported a variety of often study-specific outcomes, such as family dynamics and parental stress. In terms of pre-specified outcomes, broadband scales and symptom scores were the most frequently reported outcomes.
Figure 86 shows the effects on individual behaviors assessed in the studies, including showing physical aggression, externalizing problem behavior in the family, and observed ADHD behavior in a play situation.
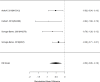
Figure 86
Effects of parent support on behavior (SMD). Notes: RE = random effects, SMD = standardized mean difference
Across studies parent interventions were associated with a positive effect on problem behavior (SMD −0.52; CI −0.85, −0.18; 4 studies, n=357). The analysis did not detect statistical heterogeneity. All included studies were RCTs. Removing one RCT judged to be high-risk of bias found a similar effect (SMD −0.47; CI −0.86, −0.08). There was some indication of publication bias (Begg p 0.08, Egger p 0.01). Using the trim and fill method for an alternative estimate found a smaller effect estimate (SMD −0.43; CI −0.63, −0.22), but the effect was still statistically significant.
Results for broadband measures are shown in Figure 87.
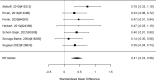
Figure 87
Effects of parent support on broadband measures (SMD). Notes: RE = random effects, SMD = standardized mean difference
Analyses found statistically significant positive effects of parent support interventions (SMD 0.41; CI 0.23, 0.58; 7 studies, n=613). The youngest children included in the studies were three years old, and the oldest were 18. The included interventions were all multi-component interventions targeting parents, but the content varied considerably. Interventions included the New Forest Parenting Package for parents of preschoolers versus wait list,110 a combination of methylphenidate plus parental training and support versus medication alone,257 a psychoeducation interventions versus treatment as usual,266 parent training for mothers versus waitlist,551 parenting strategies for preschoolers versus waitlist.325 a non-violent resistance parent training versus wait list,520 and a behavioral training for parents supported by methylphenidate versus parent education with methylphenidate treatment.569 The analysis did not detect heterogeneity. There was no evidence of publications bias. Most studies used random assignment; when restricting to RCTs only, the effect estimate was unchanged (SMD 0.42; CI 0.21, 0.64). Removing four high-risk of bias studies reported a similar point estimate but the effect was no longer statistically significant (SMD 0.52; CI −0.02, 1.05). Two of the studies reported on long-term outcomes, but estimates varied, and no meaningful summary estimate for the intervention effect could be derived (SMD 0.53; CI −2.18, 3.24; 2 studies; n=221).110, 257
A number of studies reported on ADHD symptom measures (Figure 88).

Figure 88
Effects of parent support on ADHD symptoms (SMD). Notes: ADHD = attention deficit hyperactivity disorder, RE = random effects, SMD = standardized mean difference
Analyses indicated a benefit of the parent interventions on ADHD symptoms compared to control groups not receiving the intervention, but the effect was small, and the statistical significance was borderline (SMD −0.31; CI −0.57, −0.05; 11 studies; n=1078). The youngest children included in the studies were three years old, the oldest were 18. There was little statistical heterogeneity (I-squared 52%) in results, but the multi-component interventions varied in content and complexity. Strongest effects were shown for an education and behavior strategy program for parents of preschoolers,550 psychoeducation for families,266 and the New Forest Parenting Package for parents of preschoolers,384 specifically. Most studies were RCTs; restricting exclusively to RCTs found a very similar effect estimate (SMD −0.35; CI ‑0.61, ‑0.09). Removing six high-risk of bias studies suggested a smaller, not statistically significant effect (SMD −0.31; CI −0.76, 0.14) but heterogeneity increased in this sensitivity analysis. There was some evidence of publication bias (Begg p 0.16, Egger p 0.02). Using the trim and fill method to correct for publication bias found a similar estimate (SMD −0.27; CI −0.52, −0.03), which was still statistically significant. Three studies reported outcomes at 12 months or more; there was no systematic effect across studies (SMD −0.02; CI −0.71, 0.67; 3 studies; n=324).228, 257, 290 One study evaluating an education and behavior strategy program for parents of preschoolers reported on a categorical symptom outcome; the study found no statistically significant effect (RR 2.13; CI 0.93, 4.89; 1 study, n=50).550
Functional impairment outcomes were also frequently reported in identified studies, as shown in Figure 89.
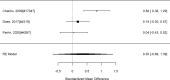
Figure 89
Effects of parent support on functional impairment (SMD). Notes: RE = random effects, SMD = standardized mean difference
Pooled effect estimates showed no systematic effect of the intervention on functional impairment (SMD 0.35; CI −0.69, 1.39; 3 studies, n=252). There was some heterogeneity (I-squared 71%). Removing one high risk of bias study reported also a non-significant effect with wide confidence intervals (SMD 0.44; CI −4.60, 5.49). There was no evidence of publication bias. One of the studies reported a long-term effect, which was not statistically significant (SMD 0.19; CI −0.20, 0.57; 1 study, n=103).228
There were insufficient data to calculate effects on treatment satisfaction, academic outcomes, appetite suppression, and number of participants with adverse events.
5.3.11.1. Parent Support Comparative Effectiveness
Multiple studies reported comparative effects, usually comparing two different parenting approaches.
Two studies assessed the New Forest Parenting program compared to an alternative approach. One study compared the New Forest Parenting versus an alternative comprehensive program (Helping the Noncompliant Child) and found no difference in aggressive behaviors (SMD 0.05; CI −0.29, 0.40; 1 study, n=164) but the CPRS ratings were lower in the Helping the Noncompliant Child group (SMD −0.41; CI 0.76, −0.07; 1 study, n=164). There was no difference in treatment satisfaction (SMD −0.13; CI −0.48, 0.21; 1 study, n=164).110 One of the studies compared Helping the Noncompliant Child to methylphenidate treatment plus sham parent training.569 The study found no statistically significant difference between intervention arms for a broadband measure (SMD −0.14; CI −0.53 0.25; 1 study, n=102) or ADHD symptom scale scores (SMD 0.06; CI −0.32, 0.45; 1 study, n=102). The effect estimates for appetite suppression (RR 0.78; CI 0.38, 1.62; 1 study, n=101) and the number of participants with adverse events (RR 0.97; CI 0.86, 1.10; 1 study, n=99) was also not statistically significant. One study compared the New Forest Parenting program with the Incredible Years alternative parenting program.552 The study found no difference in ADHD symptom scores (SMD −0.09; CI −0.33, 0.15; 1 study, n=307). A study by the same author group compared a parent training focusing on education about ADHD and behavior management strategies versus a parent counseling and support intervention.550 The study found no differences in effects on behavior in direct observations (SMD 0.36; CI −0.36, 0.88; 1 study, n=307) or broadband measure scores (RR 0.74; 0.42, 1.30; 1 study, n=307), but results statistically significantly favored the parent training when comparing the parental ratings of childhood symptom scores to assess ADHD (SMD −0.69; CI −1.22, −0.16; 1 study, n=307).
A study comparing parent psychoeducation to parent counseling found no statistically significant differences in ADHD symptom assessments (SMD −0.32; CI −0.77, 0.13; 1 study, n=81) or functional impairment (SMD 0.07; CI −0.38, 0.52; 1 study, n=81), and concluded that psychoeducation is a complementary rather than a substitute treatment.265
A study (n=92) evaluating a behavioral parent training for children with ADHD targeting executive function versus a consequence-based program did not report sufficient detail on our key outcomes to calculate effect sizes, but the study concluded positive effects on daily rated problem behaviors and hyperactivity-impulsivity symptoms for both interventions. Results favored the targeted behavioral training for inattention. A nursing case-management intervention working with families versus receiving a parenting book and newsletter did not report sufficient detail to assess effect sizes but the study (n=174) indicated that for broadband measures there were no significant differences between groups (while the overall evaluation was considered positive).200 A study comparing a parental friendship coaching intervention versus psychoeducation and social support found no significant differences in aggressive behaviors in the children with ADHD (SMD 0.14; CI −0.16, 0.43; 1 study, n=172), but the study concluded that the coaching intervention showed parents providing more emotion strategies and praise.544
Authors comparing the STEPP (Strategies To Enhance Positive Parenting) program to a traditional parent training program found no differences in ADHD symptoms (SMD 0.16; CI ‑0.28, 0.60; 1 study, 120) but found lower functional impairment scores favoring STEPP (SMD 0.51; CI 0.07, 0.96; 1 study, n=120).176
One study compared behavior parent training in a group versus individual training plus education; it found no statistically significant difference in effects on ADHD symptom scores (SMD −0.24; CI −0.77, 0.28; 1 study; n=56).593
5.3.11.2. Parent Support Summary of Findings
Table 22 shows the findings for the outcomes of interest together with the number of studies and study identifiers.
Table 22
KQ2 summary of findings and strength of evidence for parent support.
Across studies, parent training interventions were associated with improvements in broadband measure scores (moderate strength of evidence, downgraded for the domain inconsistency, given the variation in intervention approaches and the lack of replication of intervention effects. Standardized ADHD symptom scores (low strength of evidence) was downgraded for imprecision, given that the pooled effect was very close to a statistically non-significant result. There was no systematic effect on individual behaviors assessed in the studies, but the existing evidence is limited (inconsistency). We found no systematic effect on functional impairment, but we downgraded for the domain inconsistency as effect estimates varied. Evidence was insufficient to determine acceptability of treatment, academic performance, appetite suppression, and participants with adverse events due to lack of research reporting on the outcome (downgraded for inconsistency as no replication could be evaluated).
The strength of evidence for comparative studies was insufficient as studies had not been replicated yet and all results were unique to the reported study and the robustness of results could not be further evaluated; in addition, it was unclear whether the study was sufficiently powered to detect a difference for the outcome examined.
5.3.12. School Interventions
We identified ten studies reporting on teacher or school environment interventions.163, 208, 238, 259, 433, 529, 531, 577, 602, 640 The earliest study was published in 2009.602 Interventions were evaluated in three different countries, predominantly the United States. The populations studied were most often children attending elementary through middle school between the ages of 6 and 14, with only one study including adolescents up to 17 years old.521 In two studies, participants were required to demonstrate an IQ of 80 or higher.163, 259 Only one study required participants to not be taking stimulant medication or to be on a stable dose with no plans of change during the study duration.208 The majority of participants used ADHD medication at baseline. For studies that provided information on ADHD presentations, the combined type was the most prevalent presentation, followed by inattentive type. While ADHD participants with co-occurring disorders were not excluded from most of the studies, one study purposely required participants to have word-reading difficulties or reading disabilities in addition to ADHD.577 Several studies also report on participant co-occurring disorders, with the most common conditions reported being oppositional defiant disorder, conduct disorder, and anxiety and mood disorders.163, 529, 531, 577, 602
More than half of the studies used a multimodal intervention strategy comprising both teacher training and parent training,163, 529, 531, 577, 640 or included intervention components targeting the children with ADHD.163, 238, 259, 433, 531, 577 Two studies examined teacher-specific interventions. One208 tested a Web-based online learning modules for elementary-school teachers, while the other602 tested two different types of ADHD consultation services for teachers to help them plan and execute classroom-based ADHD interventions for students. Most studies reported on a control group, including waitlist control, no intervention, ADHD medication only (compared to other modes of active treatment),529, 640 and treatment as usual. Some studies reported on an alternative intervention, such a lower intensity intervention531 or a modified version of an original intervention259 and evaluated the comparative effectiveness of these interventions.
Studies reported a variety of often study-specific outcomes, such as improvement in individual cognitive tasks. In terms of pre-specified outcomes, symptom scores, functional impairment, and academic scores were the most frequently reported outcomes.
Two studies reported on individual problem behaviors, but results were conflicting and could not be combined to a meaningful summary estimate (SMD −0.01; CI −1.38, 1.36; 2 studies, n=395).238, 531 One of these reported on a long-term outcome: an evaluation of an intensive summer program reported no differences in school disciplinary incidents compared to no intervention (SMD 0.09; CI −0.18, 0.36; 1 study, n=209) at the 12 month follow up.531
We did not identify studies reporting on broadband measure scores to assess the effect of a school intervention.
Studies reporting on ADHD symptoms are shown in Figure 90.
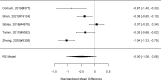
Figure 90
Effects of school interventions on ADHD symptoms (SMD). Notes: ADHD = attention deficit hyperactivity disorder, RE = random effects, SMD = standardized mean difference
Across studies, we did not find a systematic effect of school interventions on ADHD symptoms (SMD −0.50; CI −1.05, 0.06; 5 studies, n=822). The age of the children in the included studies ranged from six to 17. There was evidence of heterogeneity (I-squared 87%). We found no indication of publication bias. Removing high-risk of bias studies in a sensitivity analysis left only three studies; the effect estimate was smaller and was also not statistically significant (SMD −0.15; CI −2.99, 2.68). Heterogeneity was only marginally reduced. One of the studies reported on a long-term outcome: an evaluation of an intensive summer program reported no differences in ADHD symptoms (SMD 0.07; CI −0.20, 0.34; 1 study, n=282) at the 12 month follow up.531
Two studies assessed the effects on functional outcomes, however, they reported conflicting results and could not be combined to a meaningful summary estimate (SMD 0.22; CI −4.39, 4.82; 2 studies; n=274).208, 259 There was heterogeneity (I-squared 83%) but no further analyses could be performed due to the small number of studies. One of the studies evaluated a Web-based intervention for teachers of elementary students with ADHD208 and reported improvements in functional impairment in the students. The other assessed a school-based training intervention program for adolescents but found no differences compared to community care in a peer relation rating scale at the 12-month follow up.259
Three studies reported favorable results regarding the acceptability of the treatment approach, but there was insufficient data to compute effect sizes.208, 529, 531
A small number of school intervention studies reported on academic performance measures as shown in Figure 91.

Figure 91
Effects of school interventions on academic performance (SMD). Notes: RE = random effects, SMD = standardized mean difference
Although most individual studies reported some improvement, across studies, the effect was not statistically significant (SMD −0.19; CI −0.48, 0.09; 5 studies, n=854). There was little heterogeneity (I-squared 53%). We did not detect potential publication bias. Removing one high-risk of bias study found a smaller effect that was not statistically significant (SMD −0.10; CI 0.33, 0.12) and the analysis detected no heterogeneity, suggesting that methodological rigor of the studies was a source of heterogeneity. Two of the studies reported on long-term outcomes 12 and 15 months), but the estimates varied, and neither the individual nor the combined effects were statistically significant (SMD −0.17; CI −1.69, 1.35; 2 studies, n=153).259, 531
Identified studies did not report on the other prespecified outcomes for the review appetite suppression and participants with adverse events.
5.3.12.1. School Interventions Comparative Effects
Four of the identified school interventions also reported on a comparison to an alternative intervention, all of which were also school setting interventions.
One study assessed a dose-response question and compared a high versus a low intensity summer program. The study is shown in more detail in the appendix; briefly, the authors found no differences in school disciplinary incidents (SMD 0.01; CI −0.26, 0.28; 1 study, n=325) or ADHD symptom assessments (SMD 0.01; CI −0.26, 0.29; 1 study, n=325), and they concluded that the high intensity intervention was superior only in engagement and uptake of selected skills.531
Other school interventions reported on the comparison to alternative, school-based or teacher-led interventions. This included a study comparing two homework management programs, one focused on contingency management-based treatment versus a planning skill program.163 The study found no differences in treatment acceptability (SMD 0.00; CI −0.26, 0.26; 1 study, n=222) and no statistically significant differences in GPA (grade point average) scores (SMD 0.12; CI −0.14, 0.39; 1 study, n=222) and concluded that developing a strong working alliance and engaging parents and students are key elements for school-based programs. Comparing the after-school version of the program Challenging Horizons versus the mentoring version of the program found no differences in functional impairment (SMD 0.02; CI −0.24, 0.28; 1 study, n=326) or academic performance as measured by GPA (SMD −0.19; CI −0.46, 0.07; 1 study, n=326), but the study concluded that the after school version offers more benefits for adolescents.259
One study compared approach of ongoing feedback for teachers that selected interventions for students on the basis of functional and academic assessment data versus a traditional data-based approach chosen by the teacher. The difference between interventions for academic performance was not statistically significant (SMD −0.26; CI −0.56, 0.05; 1 study, n=167).602
5.3.12.2. School Interventions Summary of Findings
Table 23 shows the findings for the outcomes of interest together with the number of studies and study identifiers.
Table 23
KQ2 summary of findings and strength of evidence for school interventions.
Several school interventions showed favorable results for key outcomes, but the pooled effects were not statistically significant and suggested no systematic effect of school interventions in general. For behavior and functional impairment, only a small number of studies was identified and these reported conflicting results so that no meaningful effect estimate could be derived and we were not able to determine whether school interventions improve these outcomes or not. Across studies, we did not find that school interventions systematically improve ADHD symptoms and although several studies found an effect on academic performance, the pooled result was not statistically significantly different from no effect and we did not detect a clear beneficial effect. Treatment acceptability (low strength of evidence) was favorable across three identified studies reporting on the outcome, but no effect estimate could be determined (downgraded for imprecision). We did not identify studies reporting on appetite suppression or participants with adverse events and no evidence statement could be derived.
The comparative effects were all rated as insufficient as none of the identified evaluations have been replicated, and all results were unique to the reported study, the specific intervention and the specific comparator; hence the robustness of results could not be evaluated.
5.3.13. Provider Interventions
We identified nine studies252, 254–256, 308, 371, 386, 451, 466 evaluating healthcare provider interventions or interventions changing how ADHD care is delivered. The earliest study was published in 2007.256 All were conducted in the United States, except for one in Canada. The patient populations studied were children with ADHD; no studies included teenagers. The percent of female participants ranged from 15 to 36 percent, where reported. Only one study386 reported ADHD presentation type; 41 percent of children were classified as inattentive, ten percent as hyperactive and 49 percent as combined presentation. No studies purposely included patients with specific co-occurring disorders. A study conducted in Philadelphia308 reported that 46 percent of patients were African American. The majority of patients in the other studies were White.
Of the identified studies, six reported on a control group with treatment as usual.252, 255, 256, 308, 386, 466 In one of these trials, pediatricians used titration trials to determine optimal medication dosages; doses were standardized by week, but doctors were blinded to exact dosage.256 Another study255 held four training sessions for providers and installed a Web portal to assist with treatment monitoring. Another combined a Web portal with an ADHD care manager.308 One study provided office-based training in using stimulant medications to physicians and one hour of training to office staff in the use of new software.386 Another created a Web-based platform that enabled clinicians to administer online clinical questionnaires to parents and teachers to monitor patients remotely between visits.466 One study evaluated the effects of pharmacogenetic testing to enable genomically assisted prescribing.252 Finally, one head to head study compared collaborative care, where a care manager delivered three or four content modules to parents and children, to enhanced usual care from a provider known to the care manager.371
The studies are difficult to compare and assessed unique interventions, often with multiple components and targeting different aspects of the healthcare system and healthcare delivery processes. In addition, many used study-specific evaluation measures and rarely reported on key outcomes prespecified for this review or did not report sufficient detail to compute effect sizes for outcomes of interest.
One study reported on a broadband measure and evaluated children under the care of providers that used a trigger algorithm and alert resolution process with online clinical questionnaires to monitor patients remotely between visits. The cluster RCT reported that the children in the intervention condition experience less improvement after 15 months in global functioning (SMD −0.36; CI −0.65, −0.07; 1 study, n=263) than the control group participants.466
Studies reported conflicting results for ADHD symptoms and no meaningful summary estimate could be derived for the intervention (SMD 0.26; CI −4.79, 5.31; 2 studies, n=537).308, 466 This included the trigger algorithm study which did not find positive effects466 and a study evaluating a care manager combined with an online electronic health record portal to enhance communication and shared decision making, which favored the intervention.308
The provider or healthcare system interventions that reported on a control group did not report on any other outcome of interest for this review. Other assessed (study-specific) outcomes are shown in evidence table C.2 in Appendix C.
5.3.13.1. Provider Interventions Comparative Effects
Two studies compared a health service intervention to an alternative model. One assessed a collaborative care model versus a referral to mental health providers in an enhanced usual care condition. The study (n=411) did not report sufficient detail to compute effect sizes but concluded that the collaborative care model improved symptoms more than the referred group.371 A telehealth service delivery model combining pharmacotherapy and caregiver behavior training versus children remaining under the care of their primary care provider who received only a single consultation with a tele-psychiatrist who shared treatment recommendations were compared in the second study.451 The study reported statistically significant improvement in symptom measures (SMD −0.54; CI −0.81, −0.27; RR 1.64; CI 1.09, 2.47; 1 study, n=223) as well as functional impairment (SMD 0.27; CI 0.01, 0.54; 1 study, n=223) for the telehealth group.451
5.3.13.2. Provider Interventions Summary of Findings
Table 24 displays the findings for the outcomes of interest together with the number of studies and study identifiers. Comparative effectiveness results are shown only for outcomes for which effect sizes could be calculated.
Table 24
KQ2 summary of findings and strength of evidence for provider interventions.
Studies targeting providers or the delivery of healthcare reported on very different intervention approaches, and studies were difficult to compare. In addition, many did not report in sufficient detail (or not at all) on the outcomes of interest for this review. All studies had moderate or high risk of bias, as randomization at the provider level led to some imbalances in patient characteristics between groups. Attrition and detection bias also affected most studies. Strength of evidence was determined to be insufficient either for lack of research (behavior, functional impairment, treatment acceptability, academic performance, appetite suppression, participants with adverse events), study limitations and lack of replication (broadband measure scores), or studies reporting conflicting results making it impossible to determine whether interventions do affect the outcomes of interest (ADHD symptoms).
All effects comparing two active interventions were based on a single study without replication and therefore determined the strength of evidence to be insufficient for evidence statements.
5.4. KQ2a. How do these outcomes vary by presentation (inattentive, hyperactive/impulsive, and combined) or other co-occurring conditions?
We assessed for all key outcomes whether the impact of interventions was associated with the ADHD presentation and whether co-occurring conditions were associated with the treatment effect. Studies varied in what proportion of children with inattentive, hyperactive/impulsive, and combined presentation of ADHD were included. Some studies targeted specific presentations, e.g., evaluated an intervention in a sample with exclusively combined presentation. And while most identified studies did not exclude children with co-occurring disorders, we identified a few studies that purposefully addressed interventions for children with specific co-occurring disorders. In these studies, all children had a dual diagnosis.
5.4.1. ADHD Presentation
Most studies included a range of ADHD presentations, although we identified one study that included only youth with inattentive ADHD presentation.476 The study evaluated an integrated psychosocial treatment approach; results are documented in Appendix C, Table C.2. A number of studies included only children with combined presentation.104, 148, 229, 261, 295, 354, 439, 444, 508, 509, 522, 567, 636 The studies evaluated diverse interventions. Half of the studies that restricted participants to the combined ADHD presentation evaluated FDA-approved pharmacologic treatments, and other individual studies assessed the effects of a behavior intervention, nutrition intervention, psychosocial interventions, neurofeedback, cognitive training, and a new pharmacological agent.
We assessed the effect of presentation in indirect comparisons across studies and we documented results of subgroup analyses as reported by the individual authors.
5.4.1.1. Indirect Analyses ADHD Presentation
We first conducted indirect analyses across the large number of studies included in the review. For individual behavior measures, we did not find an effect of the proportion of children with inattentive (p 0.10), hyperactive (p 0.44), or combined (p 0.74) presentation on the reported effect size across all included interventions. For broadband assessments, we did not find an effect on the reported effect size for the proportion of children with inattentive presentation (continuous data p 0.52, categorical data p 0.90), hyperactive (continuous data p 0.73, categorical data p 0.92), or combined (continuous data p 0.70, categorical data p 0.96) across all included interventions.
For ADHD symptom scores in studies reporting a continuous outcome, we did not find an effect on the reported effect size for the proportion of children with inattentive presentation (p 0.18), hyperactive (p 0.65), or combined (p 0.21) across all included interventions. However, the equivalent analysis for categorical outcomes was statistically significant for inattentive presentation (p 0.03). The analysis indicated that treatment effects were lower in samples with a higher proportion of inattentive children, but the effect was very small (1 percentage point increase in the inattentive proportion was associated with a 1.3% reduction in the relative risk for symptom improvement).
None of the analysis for the outcome functional impairment were significant; results were borderline for the proportion of children with inattentive presentation (p 0.12), hyperactive (p 0.31), or combined (p 0.10), indicating a systematic effect across all included interventions. Results could not be confirmed in the analyses for categorical data as too few studies were available for the analysis. There were insufficient data to test the effect for treatment satisfaction. For academic performance outcomes, results were borderline for the proportion of children with inattentive presentation (p 0.06), but results for hyperactive presentation (p 0.59) and combined presentation (p 0.25) were not statistically significant. Findings could not be confirmed nor refuted with categorical data due to lack of studies.
For the outcome appetite suppression, we did not find an effect of the presentation on the reported effect size, i.e., results for inattentive (p 0.39), hyperactive (p 0.24), or combined presentation (p 0.52) were not statistically significantly different across studies and interventions. We did not identify an effect of the likelihood of experiencing an adverse event based on the ADHD presentation as results for inattentive presentation (p 0.34), hyperactive presentation (p 0.42), and combined presentation (p 0.50) were not statistically significant.
We also analyzed this question within a more homogenous group of studies, the FDA-approved medications, i.e., the largest intervention group in the report. In this subgroup with likely less residual heterogeneity, we either found no effect of the ADHD presentation or there were too few studies for analyzes, with one exception: the proportion of participants with inattentive ADHD presentation reporting adverse events (p 0.01). When differentiating further between two large subsets, we found no effect of ADHD presentation within simulant studies or within non-stimulant studies, suggesting that the study composition and medication type may be confounded. It is unclear from these analyses whether the proportion of participants with inattentive, hyperactive, or combined presentation is systematically associated with differences in treatment effects.
5.4.1.2. Reported Analyses for Subgroups in ADHD Presentation
Some of the identified studies reported results stratified by ADHD presentation or reported results of a moderator analysis that evaluated the effects of the ADHD presentation on treatment effects. The studies reported on different intervention types including: FDA-approved pharmacological interventions,108, 164, 306, 442, 538, 557 a new pharmaceutical agent,637 psychosocial interventions;163, 523 cognitive training;166 nutritional supplements;310, 349, 411, 510 and provider training,386 respectively. The reported subgroup results were primarily for ADHD symptoms and broadband assessments.
A cognitive training intervention identified a subgroup of boys who had both a lower hyperactivity and a higher conduct disorder symptom score with significantly better planning/organizing skills than the total group of participants.166 A study evaluating an omega-3 supplement reported that improvements were significantly more frequent in the inattentive ADHD presentation (p 0.03) than in the combined ADHD presentation (no statistically significant treatment effect).349 One omega 3 and zinc study510 reported the superior effect of zinc over omega-3 was only seen in the inattentive, not in the combined presentation of ADHD children (p 0.21).
All other studies did not detect systematic effects of ADHD presentation. One study108 evaluating long-acting methylphenidate reported that inattentive and combined ADHD subgroups did not differ significantly in their improvements in the parent (p 0.61) or teacher (p 0.85) SNAP-IV ratings (Swanson, Nolan, and Pelham (SNAP) Questionnaire). A further study reported no significant treatment interaction between relapse and the ADHD presentation.164 A study evaluating atomoxetine reported that baseline ADHD severity did not moderate treatment efficacy on response inhibition (p 0.54), sustained attention (p 0.96), or fear identification (p 0.66).306 A study assessing the effects of omega 3310 found a higher percentage of children who ranked below the median in hyperactivity/impulsivity on a continuous performance test improved more in ADHD symptom severity, but the difference was not statistically significant (p 0.177). Reported results for the effects of a provider intervention on ADHD Rating Scale-IV Scores and SNAP-IV Scores showed no treatment effects specific to combined ADHD presentation or ADHD inattentive presentation.386A study of atomoxetine442 assessed changes from baseline of ADHD-RS-IV-Parent Total Score and did not find any interaction.
Some studies stratified by clinical severity. A study evaluating mixed amphetamine salts557 stratified participants by low or high baseline severity on ADHD-RS-IV Scale and CGI scores. The mean reduction in ADHD severity was greater for low baseline severity in all dose groups relative to placebo (p<0.01) on the ADHD-RS-IV scale and for doses above 10mg on CGI Impression Scores (p<0.01). In a further study, evaluated efficacy and adverse effects of methylphenidate treatment for baseline ADHD severity as reported by teachers and parents found no significant effect on parent- or teacher-rated Conners ADHD index at 16 weeks (p values >0.1).538 One study evaluating hopantenic acid386 indicated that treatment effects were maximized in patients with the ADHD combined presentation group, but between-group differences were not statistically significant. Stratified analyses of an omega 3 intervention evaluating ADHD RS-IV Scores explored whether children rated with abnormal scores Sin at least two of the Conners’ subscales showed a different treatment response. The interaction was statistically significant (p < 0.15) in four out of the eight CRS-P subscales (Parent Child Rating Scales).411 A behavioral sleep intervention for children with ADHD523 reported that children with ADHD symptom severity scores above the 75th percentile were more likely to have moderate/severe sleep problems over time. ADHD symptom severity was a moderator for ADHD symptoms (p 0.04) and quality of life (p 0.04) over time, suggesting the intervention is less effective for youth who have sleep problems. All other studies did not detect an effect.
5.4.2. Effect of Co-Occurring Disorders
We abstracted the results of study-reported effects (subgroup analyses or moderator analyses) as well as indirect comparisons across studies using a meta-regression approach.
A small number of studies addressed co-occurring disorders presenting with ADHD overall. Identified studies targeting specific populations included participants with ADHD as well as oppositional defiant disorder, conduct disorder, or aggression;151, 174, 207, 220, 226, 257, 264, 321, 432, 623 learning disabilities;221, 480, 526, 538, 577, 602, 624 sleep conditions;329, 428, 513, 523 mood disorders such as depression and anxiety;132, 292, 377 tic disorders;118, 380, 540, 556 traumatic brain injury;383 epilepsy;262 substance use disorder;497 iron deficiency;478 genetic disorders;113 or organizational deficits;106 respectively. Few of the studies reported statistically significant, systematic effects of co-occurring conditions and only selected studies reported effects on the key outcomes for this report.
In the MTA study, children with ADHD-only or ADHD with oppositional defiant disorder (ODD) or conduct disorder (but without anxiety disorders) responded best to MTA medication treatments (with or without behavioral treatments), while children with multiple comorbid disorders (anxiety and ODD/conduct disorder) responded optimally to combined (medication and behavioral) treatments;343 children with comorbid anxiety, particularly those with overlapping disruptive disorder comorbidities, showed preferential benefits to the intervention;864 no detrimental effect of anxiety on medication response for core ADHD or other outcomes in anxious or non-anxious ADHD children was demonstrated;910 comorbid anxiety disorder did moderate outcome, in participants without anxiety, results paralleled intent-to-treat findings, for those with anxiety disorders, behavioral treatment yielded significantly better outcomes than community care (and was no longer statistically different from medication management and combined treatment) regarding ADHD symptoms;942 comorbidity with oppositional defiant disorder or conduct disorder (54% of the sample yielded such preintervention comorbidity) significantly moderated findings, initial comorbidity with anxiety disorder served as a clear moderator of treatment response. Whereas the 66 percent of the MTA sample without anxiety at baseline displayed a response to treatment that was close to that of the overall sample, the 34 percent with comorbid anxiety showed a relatively better response to the behavioral aspects of the MTA treatments.830 Parent-reported anxiety and ODD/CD (oppositional defiant disorder/conduct disorder) status were noted on response to treatment, indicating that children with ADHD and anxiety disorders (but no ODD/CD) were likely to respond equally well to the MTA behavioral and medication treatments, children with ADHD-only or ADHD with ODD/CD (but without anxiety disorders) responded best to MTA medication treatments (with or without behavioral treatments), while children with multiple comorbid disorders (anxiety and ODD/CD) responded optimally to combined (medication and behavioral) treatments.863 For other functioning domains (social skills, academics, parent-child relations, oppositional behavior, anxiety/depression), results suggested slight advantages of combined over single treatments (medical management, behavior) and community care, children with parent-defined comorbid anxiety disorders, particularly those with overlapping disruptive disorder comorbidities, showed preferential benefits to the behavioral and combined interventions.864 A further study461 reported that youths with ADHD and comorbid ODD showed statistically significant improvement in ADHD, ODD, and quality-of-life measures following atomoxetine treatment; treatment response was similar in youths with and without ODD, except that the comorbid group showed improvement compared with placebo at 1.8 mg/kg/day but not 1.2 mg/kg/day. In contrast, youths without ODD showed improvement at 1.2 mg/kg/day and no incremental benefit at 1.8 mg/kg/day. A third study reported that children with ODD did not benefit as much from the atomoxetine than other children.133 One study enrolled children with ADHD and aggressive behavior and titrated stimulant treatment to identify patients with inadequate reductions in aggressive behavior. The study concluded that rigorous titration of stimulant medication and concurrent behavioral therapy may avert the need for additional medications.151 All other studies did not detect treatment effect differences associated with co-occurring conditions or reported on other outcomes such as ODD scores as documented in Appendix C, Table C.3.
5.4.2.1. Indirect Analyses, Co-Occurring Disorders
We assessed whether the subgroup influences the impact of the interventions for the key outcomes in indirect comparisons. For the outcome behavior, we did not find a systematic effect across any of the evaluated subgroups that provided sufficient data for the analysis (sleep p 0.99). For broadband scale scores, we also found no systematic effect (sleep p 0.07). Symptom scores provided the most data for the comparisons; however, the analysis did not detect systematic effects (sleep p 0.50). For functional outcomes, results were also not statistically significant (sleep p 0.93). Treatment satisfaction could not be evaluated due to the small number of studies. Appetite suppression was not significant (learning disability p 0.41), nor was adverse events (sleep p 0.68).
Within the more homogenous subgroup of FDA-approved medications, stimulants alone, and non-stimulants alone, there were insufficient data for analyses for all outcomes of interest.
We did not detect evidence indicating a differential effect associated with co-occurring disorders. However, based on the small number of studies and the indirect nature of effect analysis, the results have to be interpreted with caution.
5.6. KQ2b. What is the risk of diversion of pharmacologic treatment?
Only two studies met inclusion criteria for KQ2b.455, 497 One was an RCT evaluating either 200 or 400 mg viloxazine vs placebo and found no evidence for misuse.455 Viloxazine, however, is a non-stimulant (NRI) medication with low abuse potential.
The other study was a double-blind RCT of OROS (Osmotic-Release Oral System) methylphenidate plus cognitive behavioral therapy (CBT) versus placebo plus CBT in adolescents with ADHD and a co-occurring substance use disorder.497 Rates of misuse or diversion in the stimulant group (2.1%-4.8%) were approximately double the rates in the placebo group, though the differences did not reach statistical significance. Findings are difficult to generalize to non-substance-use ADHD populations, as misuse and diversion rates may be higher in this subpopulation than in ADHD adolescents without substance use disorder. However, nearly doubled rates of misuse may be clinically relevant, given that participants were blinded to treatment assignment, and rates were systematically higher in the stimulant group.
5.7. Summary of Findings KQ2a and KQ2b
Table 25 documents the results across studies.
Table 25
KQ2a summary of findings and strength of evidence for ADHD interventions.
Across identified studies, we either detected no evidence of effect modifiers or the research base was insufficient for any evidence statements. We downgraded results for indirectness given that the comparison was indirect, across studies. In several instances, we also downgraded for the domain inconsistency because consistency could not be assessed or could not be assumed because the identified studies did not cover the entire range of possible variables (e.g., a small number of studies only addressed co-occurring disorders systematically).
We identified only a small number of studies that systematically addressed co-occurring disorders, and evidence is insufficient for concrete evidence statements. Only two studies reported on diversion, and it was therefore not possible to quantify the risk of diversion of pharmacological treatment.
- KQ2, ADHD Treatment Key Points
- KQ2, ADHD Treatment Results
- Effects by Intervention
- KQ2a. How do these outcomes vary by presentation (inattentive, hyperactive/impulsive, and combined) or other co-occurring conditions?
- KQ2b. What is the risk of diversion of pharmacologic treatment?
- Summary of Findings KQ2a and KQ2b
- Results: Treatment of ADHD - ADHD Diagnosis and Treatment in Children and Adoles...Results: Treatment of ADHD - ADHD Diagnosis and Treatment in Children and Adolescents
Your browsing activity is empty.
Activity recording is turned off.
See more...