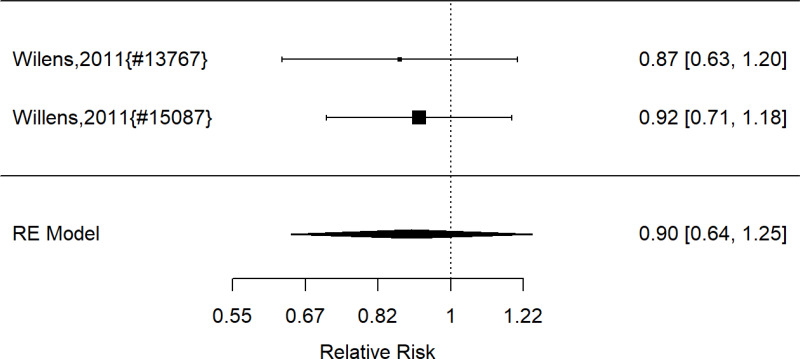
From: 5, Results: Treatment of ADHD

ADHD Diagnosis and Treatment in Children and Adolescents [Internet].
Comparative Effectiveness Review, No. 267.
Peterson BS, Trampush J, Maglione M, et al.
Rockville (MD): Agency for Healthcare Research and Quality (US); 2024 Mar.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.