NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nifedipine is a first generation calcium channel blocker used to treat hypertension and angina pectoris. Nifedipine therapy is associated with a low rate of serum enzyme elevations and has been linked to several instances of clinically apparent acute liver injury.
Background
Nifedipine (nye fed' i peen) belongs to the dihydropyridine class of calcium channel blockers (first in its class and similar to amlodipine, felodipine and nicardipine) and is used for the treatment of hypertension and angina pectoris. Like other calcium channel blockers, nifedipine acts by inhibiting the transmembrane influx of calcium into cardiac and smooth muscle cells during depolarization. The inhibition of calcium influx causes arterial vasodilation and decreases cardiac work and oxygen consumption. Nifedipine was approved in the United States in 1982 and currently several million prescriptions are filled yearly. Current indications include hypertension and chronic stable and Prinzmetal's variant angina pectoris. It is also used off label to treat Raynaud phenomenon. Nifedipine is available in capsules of 10 and 20 mg and as extended release tablets of 30, 60 and 90 mg in several generic formulations as well as under brand names including Procardia, Adala, Afeditab, Nifediac, and Nifedical. The typical dose in adults is 30 to 60 mg daily, usually starting with lower doses. Nifedipine is generally well tolerated and side effects are largely due to its vasodilating activities and can include dizziness, flushing, headache, fatigue, nausea, diarrhea, palpitations, bradycardia, peripheral edema and skin rash.
Hepatotoxicity
Mild and transient elevations in serum aminotransferase levels may occur during nifedipine therapy, but often resolve even with continuation of therapy. Clinically apparent acute liver injury with jaundice due to nifedipine is rare and described only in isolated case reports. The time to onset of injury is typically 1 to 2 months and the pattern of serum enzyme elevations is usually hepatocellular or mixed. Rash, arthralgias, fever and eosinophilia can occur, but are not prominent. Chronic aminotransferase elevations during continued therapy with nifedipine have been described sometimes with histological features of alcoholic liver disease (steatosis and Mallory bodies), but chronic liver injury after withdrawal has not. Nifedipine has not been implicated in cases of vanishing bile duct syndrome or acute liver failure in the published literature.
Likelihood score: B (likely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of nifedipine hepatotoxicity is not known, but is likely to be due to production of a toxic or immunogenic intermediate during its metabolism by the liver.
Outcome and Management
The severity of liver injury from nifedipine ranges from mild and transient serum enzyme elevations to self-limited jaundice to an alcoholic hepatitis-like syndrome. Complete recovery is expected after stopping the drug and recovery is usually rapid (3 to 8 weeks). There is little information on cross sensitivity to liver injury among the various calcium channel blockers, but related medications should be started with caution in patients with clinically apparent liver injury from nifedipine.
Drug Class: Cardiovascular Agents, Calcium Channel Blockers
Other Drugs in the Subclass, Calcium Channel Blockers: Amlodipine, Diltiazem, Felodipine, Isradipine, Nicardipine, Nimodipine, Nisoldipine, Verapamil
CASE REPORT
Case 1. Acute hepatitis-like syndrome attributed to amlopidine.
[Modified from: Basile C, Mascia E. Dihydropyridine calcium channel blockers: a rare and reversible cause of hepatotoxicity with cholestasis in a CAPD patient. Nephrol Dial Transplant 1999; 14: 2776-7. PubMed Citation]
A 76 year old man with diabetes and end stage renal disease developed jaundice while on long term nifedipine therapy (60 mg daily for ~3 years) for hypertension. He was taking insulin, but no other medications. He had no risk factors for viral hepatitis and did not drink alcohol. Serum bilirubin was 2.5 mg/dL and rose over the next few months to 6.2 mg/dL. Tests for acute hepatitis A, B and C were negative and abdominal ultrasonography showed a normal liver and gallbladder. Nifedipine was stopped and he recovered rapidly. Several months later, amlodipine was started (10 mg daily) and within 6 weeks, he developed jaundice and a cholestatic pattern of serum enzyme elevations. Once amlodipine was stopped, liver tests improved and were normal three weeks later.
Key Points
| Medication: | Nifedipine (60 mg daily) and then amlodipine (10 mg daily) |
| Pattern: | Cholestatic (R=0.3 and 0.8 during recurrence) |
| Severity: | 2+ (jaundiced, but not hospitalized for liver injury) |
| Latency: | 3 years for nifedipine, 2 months for amlodipine |
| Recovery: | 3 to 4 weeks |
| Other medications: | Insulin |
Laboratory Values
| Date | Duration Therapy | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|---|
| Long term therapy with nifedipine (60 mg daily) | ||||||
| May 12, 1998 | 3 years | 13 | 144 | 0.3 | ||
| Sep 9, 1998 | 47 | 783 | 0.5 | |||
| Dec 9, 1998 | 40 | 955 | 2.5 | |||
| Jan 11, 1999 | 3.5 years | 0 | 79 | ... | 6.2 | |
| Nifepidine stopped January 11, 1999 | ||||||
| Jan 16, 1999 | 1 week | 46 | 531 | 4.0 | ||
| Jan 23, 1999 | 2 weeks | 45 | 419 | 1.8 | ||
| Feb 11, 1999 | 4 weeks | 17 | 185 | 0.9 | ||
| Amlodipine (10 mg daily) started in late March, 1999 | ||||||
| Apr 10, 1999 | 2 weeks | 43 | 398 | 0.6 | ||
| May 5, 1999 | 6 weeks | 0 | 74 | 398 | 6.3 | |
| Amlodipine stopped May 7, 1999 | ||||||
| May 11, 1999 | 4 days | 70 | 399 | 5.4 | ||
| May 13, 1999 | 6 days | 54 | 314 | 4.1 | ||
| May 28, 1999 | 3 weeks | 19 | 181 | 1.3 | ||
| Normal Values | <55 | <250 | <1.2 | |||
Comment
One of the few reports of acute liver injury with jaundice attributed to calcium channel blockers. The patient initially developed a cholestatic hepatitis while on long term nifepidine therapy. Liver tests because steadily worse and nifepidine was ultimately withdrawn, whereupon liver tests fell to normal within 4 weeks. There was a recurrence of liver injury within 2 to 6 weeks of restarting another calcium channel blocker, with a similar pattern of liver test abnormalities and a similar rapid improvement upon stopping. Both nifedipine and amlodipine are dihydropyridines, but there structures are dissimilar.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nifedipine – Generic, Adalat®, Procardia®
DRUG CLASS
Cardiovascular Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nifedipine | 21829-25-4 | C17-H18-N2-O6 |
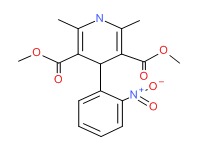 |
ANNOTATED BIBLIOGRAPHY
References updated: 11 January 2017
- Zimmerman HJ. Calcium channel blockers. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 646-7.(Expert review of hepatotoxicity published in 1999; among calcium channel blockers, diltiazem, nifedipine, bepridil and verapamil have been incriminated in instances of hepatic injury).
- De Marzio DH, Navarro VJ. Calcium channel blockers. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 524.(Review of hepatotoxicity of calcium channel blockers mentions that diliazem, nefidipine and verapamil have been implicated in causing cholestatic liver injury in a small number of patients).
- Michel T, Hoffman BB. Calcium channel antagonists. Treatment of myocardial ischemia. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 755-60.(Textbook of pharmacology and therapeutics).
- Rotmensch HH, Roth A, Liron M, Rubinstein A, Gefel A, Livni E. Lymphocyte sensitization in nifedipine-induced hepatitis. Br Med J 1980; 281; 976-7. [PMC free article: PMC1714362] [PubMed: 7000285](69 year old man developed fever and jaundice 10 days after starting nifedipine [bilirubin 3.0 mg/dL, AST 56 U/L, Alk P 420 U/L, ESR 95 mm/hr], with rapid resolution and recurrence of fever and Alk P elevations after rechallenge with a single dose).
- Davidson AR. Lymphocyte sensitization to nifedipine-induced hepatitis. Br Med J 1980; 281: 1354. [PMC free article: PMC1714796] [PubMed: 7437797](Letter in response to Rotmensch [1980] with a similar case: 59 year old man developed fatigue 2 weeks after starting nifedipine [bilirubin 3.0 mg/dL, ALT 49 U/L, Alk P 1.5 times ULN], resolving rapidly upon stopping).
- Abramson M, Littlejohn GO. Hepatic reactions to nifedipine. Med J Aust 1985; 142: 47-8. [PubMed: 3965874](Two cases: 65 year old man developed jaundice 6 weeks after starting nifedipine [bilirubin 1.8 mg/dL, ALT 1220 U/L, Alk P 1094 U/L], resolving within 1 month and later tolerating verapamil; 72 year old man developed fever and arthralgias 6 weeks after starting verapamil [bilirubin normal; ALT 100 U.L, Alk P 457 U/L], resolving in a few weeks).
- Welch HG, Lazar B, Gresser J, McMahon BJ. Nifedipine-induced hepatitis. Alaska Med 1986; 28:11-2. [PubMed: 3717520](50 year old woman with myocardial ischemia developed AST elevations [31 rising to 361 U/L] within 4 days of starting nefidipine, with no change in bilirubin [1.0 mg/dL] and rapid resolution when it was stopped).
- Kiire CF, Rutherford D. Nifedipine-associated jaundice: a second case. East Afr Med J 1986; 63: 560-1. [PubMed: 3792245](75 year old man developed jaundice and abdominal pain 2 weeks after starting nifedipine [bilirubin 3.5 mg/dL, AST 53 U/L, Alk P 139 U/L], resolving within 2 months of stopping).
- Richter WO, Schwandt P. Serious side effects of nifedipine. Arch Intern Med 1987; 147: 1852. [PubMed: 3662717](69 year old woman developed marked Alk P elevations [943 U/L] with normal bilirubin [1.5 mg/dL], with no change after stopping prazosin and allopurinol, but prompt decrease after stopping nifedipine and recurrence on inadvertent rechallenge [Alk P 529 U/L]).
- Shaw DR, Misan GM, Johnson RD. Nifedipine hepatitis. Aust N Z J Med 1987; 17: 447-8. [PubMed: 3435325](80 year old woman developed nausea and abdominal pain 4 days after starting nifedipine and having an episode of shock [bilirubin 2.0 mg/dL, AST 1065 U/L, LDH 1190 U/L, Alk P 352 U/L], resolving within 8 weeks).
- Biour M, Grange JD, Barbare JC, et al. [Hepatic involvement due to nifedipine. Description of a case and review of the literature] Therapie 1987; 42: 301-3 [French]. [PubMed: 3660324](60 year old man developed jaundice 10 days after starting nifedipine [bilirubin 16.1 mg/dL, ALT 36 times ULN, Alk P 2.3 times ULN], resolving within one month of stopping).
- Babany G, Uzzan F, Larrey D, et al. Alcohol-like liver lesions induced by nifedipine. J Hepatol 1989; 9: 252-5. [PubMed: 2809167](78 year old woman taking nifedipine developed mild elevations of Alk P [1.5 times ULN] and GGT [5.3 times ULN] and underwent liver biopsy that showed steatohepatitis and Mallory bodies, and had ascites 6 months later despite normal enzymes; repeat biopsy showed fibrosis and fat without Mallory bodies; no follow up after stopping nefidine was given).
- Sawaya GF, Robertson PA. Hepatotoxicity with the administration of nifedipine for treatment of preterm labor. Am J Obstet Gynecol 1992; 167: 512-3. [PubMed: 1497061](25 year old pregnant woman developed early uterine contractions and was treated with terbutaline and nifedipine whereupon ALT rose to as high as 845 U/L with no symptoms or jaundice, falling to normal 2 weeks after delivery and stopping nifedipine).
- Basile C, Mascia E. Dihydropyridine calcium channel blockers: a rare and reversible cause of hepatotoxicity with cholestasis in a CAPD patient. Nephrol Dial Transplant 1999; 14: 2776-7. [PubMed: 10534534](76 year old man developed jaundice 3 years after starting nifedipine [bilirubin 6.2 mg/dL, ALT 79 U/L, Alk P 955 U/L], resolving within 1 month of stopping and recurring within 6 weeks of starting amlodipine [bilirubin 6.3 mg/dL, ALT 74 U/L, Alk P 398 U/L] resolving in 3 weeks: Case 1).
- Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, Ruilope LM. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000; 356: 366-72. [PubMed: 10972368](Randomized clinical trial of nifedipine versus diuretics for an average of 3 years in 6321 patients reported similar outcomes and no differences in adverse events; liver toxicity not mentioned).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but none were attributed to calcium channel blockers).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719](Summary of 25 years of adverse drug reaction reporting in Sweden identified 103 cases of drug induced acute liver failure; only one case was possibly linked to a calcium channel blocker: felodipine).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, calcium channel blockers were implicated as a sole agent in 2 cases [1 amlodipine, 1 verapamil] and as one of several agents in 2 cases [both amlodipine]).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were linked to calcium channel blockers).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 114: 1419-25. PubMed Citation (In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but no cases were attributed to calcium channel blockers).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases; one case was attributed to verapamil, but none were linked to diltiazem or other calcium channel blockers).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 39 [4%] were due to antihypertensive agents including 4 due to calcium channel blockers [amlodipine in 1 and verapamil in 3 instances], but none to diltiazem).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Verapamil.[LiverTox: Clinical and Researc...]Review Verapamil.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Amlodipine.[LiverTox: Clinical and Researc...]Review Amlodipine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Nicardipine.[LiverTox: Clinical and Researc...]Review Nicardipine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Calcium Channel Blockers.[LiverTox: Clinical and Researc...]Review Calcium Channel Blockers.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Diltiazem.[LiverTox: Clinical and Researc...]Review Diltiazem.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Nifedipine - LiverToxNifedipine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...