NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
The calcium channel blockers act by blocking the influx of calcium ions into vascular smooth muscle and cardiac muscle cells during membrane depolarization. Because muscle contraction is largely dependent upon influx of calcium, its inhibition causes relaxation, particularly in arterial beds. Thus, the major effects of the calcium channel blockers are relaxation of vascular and arterial smooth muscle cells resulting in arterial vasodilation. The major use of the calcium channel blockers is for hypertension and angina pectoris (variant, exertional, and unstable). Some calcium channel blockers are also used for supraventricular arrhythmias and heart failure. Off label uses include migraine headaches. The major calcium channel blockers used in the United States include amlodipine, diltiazem, felodipine, isradipine, nicardipine, nifedipine, nimodipine, nisoldipine, and verapamil. While all affect the L type voltage gated calcium channel, the structure and site of interaction within the channel varies among the agents. Verapamil blocks the phenylalkylamine site and diltiazem the benzothiazepine site, while the remaining agents (exemplified by amlopidine and nifedipine) bind to the 1,4 dihydropyridine site. These agents are also commonly referred to as being first generation (verapamil, diltiazem, nifedipine) or second generation (amlopine, felodipine, isradipine, nicardipine, nimodipine and others) calcium channel blockers. Several of the calcium channel blockers are now available in generic forms and some are available as combinations with diuretics and lipid lowering agents.
Many of the calcium channel blockers have been linked to rare instances of idiosyncratic drug induced liver disease. Hepatic injury from calcium channel blockers is usually mild and reversible, but rare symptomatic and severe instances have been reported. Agents most clearly linked to liver injury are verapamil, diltiazem, amlodipine and nifedipine, probably because these agents have been most widely used. The pattern of injury varies somewhat among the different agents, so that the hepatic injury appears not to be a class effect or the result of inhibition of calcium channels, but rather due to hypersensitivity (verapamil) or metabolic injury that usually results in a mixed hepatocellular-cholestatic pattern (diltiazem, amlopidine, nifedipine). These four agents have different chemical structures and interact with different sites or different calcium channels, so that different patterns of idiosyncratic adverse events and different hepatic reactions might be expected. However, repeated instances of liver injury from different calcium channel blockers have been reported, so that switching from one calcium channel blocker that has caused liver injury to another in this class should be done with caution. Several of these agents can also cause minor elevations in serum aminotransferase levels that are often transient and resolve even with continuation of the agent or can be persistent, resolving only once the agent(s) are discontinued. Common, minor class specific side effects of the calcium channel blockers include headache, dizziness, flushing, nausea, fatigue, diarrhea, peripheral edema, palpitations, bradycardia and rash.
Each calcium channel blocker is discussed separately with individual clinical cases and references. The following are links to each drug record.
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Amlodipine | 88150-42-9 | C20-H25-C1-N2-O5 |
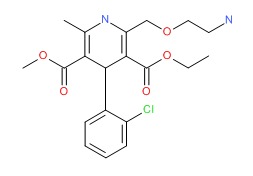 |
| Diltiazem | 42399-41-7 | C22-H26-N2-O4-S |
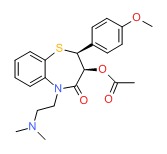 |
| Felodipine | 72509-76-3 | C26-H29-N3-O6 |
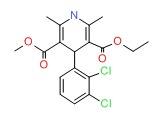 |
| Isradipine | 75695-93-1 | C19-H21-N3-O5 |
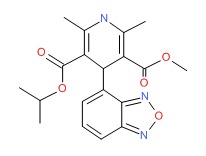 |
| Nicardipine | 55985-32-5 | C26-H29-N3-O6 |
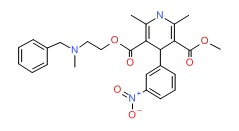 |
| Nifedipine | 21829-25-4 | C17-H18-N2-O6 |
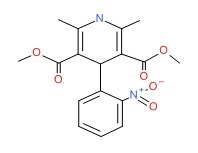 |
| Verapamil | 52-53-9 | C27-H38-N2-O4 |
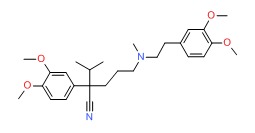 |
ANNOTATED BIBLIOGRAPHY
References Last Updated: 11 January 2017
- Zimmerman HJ. Calcium channel blockers. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 646-7.(Expert review of hepatotoxicity published in 1999; among calcium channel blockers, diltiazem, nifedipine, bepridil and verapamil have been incriminated in instances of hepatic injury).
- De Marzio DH, Navarro VJ. Calcium channel blockers. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 524.(Review of hepatotoxicity of the calcium channel blockers mentions that diltiazem, nifedipine and verapamil have been implicated in causing serum aminotransferase elevations and steatohepatitis).
- Eschenhagen T. Treatment of hypertension. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 507-26.(Textbook of pharmacology and therapeutics).
- Andrade RJ, Lucena MI, Fernández MC, et al.; Spanish Group for the Study of Drug-Induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005; 129: 512-21. [PubMed: 16083708](Reports to a Spanish network found 461 cases of drug induced liver disease; no calcium channel blocker mentioned among the 19 most common causes [those associated with 5 cases or more]).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but none were attributed to a calcium channel blocker).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719](Summary of 25 years of adverse drug reaction reporting in Sweden identified 103 cases of drug induced acute liver failure; only one case was possibly linked to a calcium channel blocker--felodipine).
- Triggle DJ. Calcium channel antagonists: clinical uses--past, present and future. Biochem Pharmacol 2007; 74: 1-9. PubMed Citation (Review of the efficacy and safety of calcium channel blockers and their changing roles in therapy of hypertension and angina pectoris).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. PubMed Citation. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, calcium channel blockers were implicated as a sole agent in 2 cases [1 amlodipine, 1 verapamil] and as one of several agents in 2 cases [both amlodipine]).
- Chaudhry M, Maqsood A, Diab-Agha S, Rosenberg J. Nicardipine-induced acute hepatitis in an intensive care unit patient. Am J Ther 2009 16: 71-3. [PubMed: 19092642](62 year old man with acute myocardial infarction given nicardipine intravenously, developed fever and serum enzyme elevations 4 days later with ALT 356 U/L, Alk P 299 U/L, but normal bilirubin, resolving within days of stopping).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, none of which were attributed to calcium channel blockers).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, of which none were attributed to calcium channel blockers).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases; one case was attributed to verapamil, but none were linked to other calcium channel blockers).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 39 [4%] were due to antihypertensive agents including 4 due to calcium channel blockers [amlodipine in 1 and verapamil in 3 instances]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Calcium channel antagonists. Part III: Use and comparative efficacy in hypertension and supraventricular arrhythmias. Minor indications.[Cardiovasc Drugs Ther. 1988]Review Calcium channel antagonists. Part III: Use and comparative efficacy in hypertension and supraventricular arrhythmias. Minor indications.Opie LH. Cardiovasc Drugs Ther. 1988 Mar; 1(6):625-56.
- In vivo characterization of combination antitumor chemotherapy with calcium channel blockers and cis-diamminedichloroplatinum(II).[Cancer Res. 1989]In vivo characterization of combination antitumor chemotherapy with calcium channel blockers and cis-diamminedichloroplatinum(II).Onoda JM, Nelson KK, Taylor JD, Honn KV. Cancer Res. 1989 Jun 1; 49(11):2844-50.
- Review Update on calcium-channel blocking agents.[Clin Pharm. 1983]Review Update on calcium-channel blocking agents.Talbert RL, Bussey HI. Clin Pharm. 1983 Sep-Oct; 2(5):403-16.
- Review Use of calcium channel antagonists for cardiovascular disease.[Am Pharm. 1993]Review Use of calcium channel antagonists for cardiovascular disease.Yedinak KC. Am Pharm. 1993 Aug; NS33(8):49-64; quiz 64-6.
- Review Differential effects of 1,4-dihydropyridine calcium channel blockers: therapeutic implications.[J Clin Pharmacol. 1987]Review Differential effects of 1,4-dihydropyridine calcium channel blockers: therapeutic implications.Katz AM, Leach NM. J Clin Pharmacol. 1987 Nov; 27(11):825-34.
- Calcium Channel Blockers - LiverToxCalcium Channel Blockers - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...