NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Isradipine is a second generation calcium channel blocker that is used to treat hypertension. Isradipine is associated with a low rate of serum enzyme elevations during therapy, but has not been linked convincingly to instances of clinically apparent liver injury.
Background
Isradipine (is rad' i peen) is an antihypertensive medication that belongs to the dihydropyridine class of calcium channel blockers. Like other calcium channel blockers, isradipine acts by blocking the influx of calcium ions into smooth muscle and cardiac cells using depolarization. This inhibition leads to vasodilation and decrease in cardiac work and oxygen consumption. Isradipine was approved in the United States in 1990 and its current sole indication is for hypertension. Isradipine is available in extended release capsules of 2.5, 5 and 10 mg generically and formerly under the commercial name DynaCirc. The recommended dose in adults is 5 to 10 mg in one or two divided doses daily. Like other calcium channel blockers, isradipine is generally well tolerated. Side effects are largely due to its vasodilating activities and include headache, dizziness, flushing, fatigue, nausea, peripheral edema and rash.
Hepatotoxicity, Outcome and Management
Isradipine is reported to be associated with mild elevations in serum aminotransferase levels during therapy that are usually transient, asymptomatic and resolve even with continued therapy. Cases of idiosyncratic liver injury attributed to isradipine have not been published. Large trials of isradipine have not mentioned liver injury, persistent serum aminotransferase elevations or discontinuation of drug because of hepatic adverse events. Liver injury has been reported with other calcium channel blockers such as verapamil, diltiazem, amlodipine and nifedipine, but only as isolated case reports and the injury was generally mild and self-limiting in outcome. Thus, clinically apparent liver injury due to isradipine must be rare, if it occurs at all.
Likelihood score: E (Unlikely cause of clinically apparent liver injury).
The reason why some calcium channel blockers are associated with liver injury while others are not is unclear. Isradipine is metabolized by the hepatic P450 system, predominantly by CYP 3A4 and is susceptible to drug-drug interactions with agents that induce or inhibit the microsomal enzyme.
Drug Class: Cardiovascular Agents, Calcium Channel Blockers
Other Drugs in the Subclass, Calcium Channel Blockers: Amlodipine, Diltiazem, Felodipine, Nicardipine, Nifedipine, Nimodipine, Nisoldipine, Verapamil
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Isradipine – Generic, DynaCirc®
DRUG CLASS
Cardiovascular Agents
Product labeling at DailyMed, National Library of Medicine, NIH
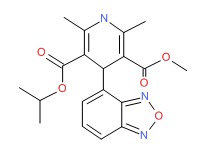
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Isradipine | 75695-93-1 | C19-H21-N3-O5 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 11 January 2017
- Zimmerman HJ. Calcium channel blockers. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 646-7.(Expert review of hepatotoxicity published in 1999; among calcium channel blockers, diltiazem, nifedipine, bepridil and verapamil have been incriminated in instances of hepatic injury; isradipine is not mentioned).
- De Marzio DH, Navarro VJ. Calcium channel blockers. Hepatotoxicity of cardiovascular and antidiabeticdrugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 524.(Review of hepatotoxicity published in 2013; diltiazem, nifedipine and verapamil have been implicated as the cause of liver injury in a small number of patients; isradipine is not specifically mentioned).
- Michel T, Hoffman BB. Calcium channel blockers. Treatment of myocardial ischemia and hypertension. In, Brunton LL, Chabner KA, Knollman KC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 766-7.(Textbook of pharmacology and therapeutics).
- Nelson EB, Pool JL, Taylor AA. Antihypertensive activity of isradipine in humans: a new dihydropyridine calcium channel antagonist. Clin Pharmacol Ther 1986; 40: 694-7. [PubMed: 2946507](Controlled trial of isradipine in 24 patients with hypertension for 3 weeks; there were no significant changes in ALT or AST values during treatment).
- Shepherd AM, Carr AA, Davidov M, Hamilton J, Schnaper H, Velasquez M, Brockway B, Prisant LM, et al. Efficacy and safety of isradipine in hypertension. J Cardiovasc Pharmacol 1989; 13: 580-5. (Among 203 patients with hypertension given placebo or one of 5 doses of isradipine for 5 weeks, [PubMed: 2470995]adverse effects were mild; 19 on isradipine [11%] and 4 on placebo [10%] had an elevation of at least one liver test, but most were transient and asymptomatic).
- British Isradipine Hypertension Group. Evaluation of the safety and efficacy of isradipine in elderly patients with essential hypertension. Am J Med 1989; 86: 110-4. [PubMed: 2523644](Among 118 elderly patients with hypertension treated with placebo or two doses of isradipine for up to 4 years, there were no differences in biochemical abnormalities between the placebo- and isradipine-treated groups).
- Dahlöf B. Hemodynamic response, safety, and efficacy of isradipine in the treatment of essential hypertension. Am J Med 1989; 86 (4A): 19-26. [PubMed: 2523651](Among 1028 patients with hypertension treated with one of 6 different doses of isradipine in 15 different studies, side effects were more frequent at higher doses and included low rates of headache, flushing, dizziness, palpitations and peripheral edema; in one studfy of 288 patients, liver test abnormalities occurred in 6.3%).
- Sundstedt CD, Rüegg PC, Keller A, Waite R. A multicenter evaluation of the safety, tolerability, and efficacy of isradipine in the treatment of essential hypertension. Am J Med 1989; 86(4A): 98-102. [PubMed: 2523665](Among 600 patients with hypertension treated with one of 3 doses of isradipine or placebo, common side effects were flushing [10.5%], headache [10.2%], and peripheral edema [4.2%]; 4 patients [0.6%] had serum aminotransferase elevations, but most were considered unrelated and all were mild, asymptomatic and did not require dose adjustment).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but none were attributed to a calcium channel blocker).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719]PubMed Citation (Summary of 25 years of adverse drug reaction reporting in Sweden identified 103 cases of drug induced acute liver failure; only one case was possibly linked to a calcium channel blocker: felodipine).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. PubMed Citation. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, calcium channel blockers were implicated as a sole agent in 2 cases [1 amlodipine, 1 verapamil] and as one of several agents in 2 cases [both amlodipine]).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, none of which were attributed to isradipine or other calcium channel blockers).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which was attributed to a calcium channel blocker or other antihypertensive medication).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases; one case was attributed to verapamil, but none were linked to isradipine or other calcium channel blockers).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 39 [4%] were due to antihypertensive agents including 4 due to calcium channel blockers [amlodipine in 1 and verapamil in 3 instances], but none to isradipine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Nicardipine.[LiverTox: Clinical and Researc...]Review Nicardipine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Felodipine.[LiverTox: Clinical and Researc...]Review Felodipine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Nifedipine.[LiverTox: Clinical and Researc...]Review Nifedipine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Calcium Channel Blockers.[LiverTox: Clinical and Researc...]Review Calcium Channel Blockers.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Calcium-channel entry blocker therapy for hypertensive patients with concomitant renal impairment: a focus on isradipine.[J Clin Pharmacol. 1994]Review Calcium-channel entry blocker therapy for hypertensive patients with concomitant renal impairment: a focus on isradipine.Frishman WH. J Clin Pharmacol. 1994 Dec; 34(12):1164-72.
- Isradipine - LiverToxIsradipine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...