NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Diltiazem hydrochloride is a first generation calcium channel blocker that is widely used in the therapy of hypertension and angina pectoris. Diltiazem therapy is associated with serum enzyme elevations and has been linked to rare instances of clinically apparent liver injury.
Background
Diltiazem (dil tye' a zem) belongs to the benzothiazepine class of calcium channel blockers and is used for the treatment of hypertension, angina pectoris and superventricular tachyarrhythmias. Like other calcium channel blockers, diltiazem acts by inhibiting the transmembrane influx of calcium ions into cardiac muscle and vascular smooth muscle cells. The inhibition of calcium flux causes arterial vasodilation and decreases cardiac work and oxygen consumption. Diltiazem, like verapamil (but unlike other calcium channel blockers), also decreases the rate of the sinus node pacemaker and slows atrial-ventricular conduction accounting for its effects on superventricular tachyarrhythmias. Diltiazem was approved in the United States in 1982 and currently several million prescriptions are filled yearly. Current indications for oral forms of diltiazem include hypertension and management of chronic stable angina pectoris, Prinzmetal's or variant angina. Diltiazem is available orally in multiple generic formulations as well as under commerical names including Cardizem and Tiazac in capsules of 30, 60, 90 and 120 mg. Once daily, extended release formulations are available and now widely used (Cardizem CD, Cardizem LA, Cartia XT, Dilacor XR, Dilt-XR, Diltia XT, Taztia XT) in strengths ranging from 60 to 420 mg. The recommended oral dose of diltiazem in adults is 180 to 360 mg daily, usually starting with lower doses. Chronic therapy is typical. Diltiazem is also available in intravenous formulations which are used in therapy of atrial arrhythmias, including atrial fibrillation or flutter and superventricular tachycardia. Diltiazem, like other calcium channel blockers, is generally well tolerated and side effects are due to its vasodilating activities and can include dizziness, flushing, headaches, fatigue, nausea, diarrhea, palpitations, bradycardia, postural hypotension and rash. Rare but potentially severe adverse events include cardiac conduction defects, hypotension, hypersensitivity reactions and instances of Stevens Johnson syndrome.
Hepatotoxicity
Diltiazem therapy is associated with a low rate of mild and transient elevations in serum aminotransferase levels which are usually asymptomatic and often resolve even with continuation of therapy. Clinically apparent, acute liver injury with jaundice due to diltiazem is rare and only isolated case reports have been published. In large case series of drug induced liver injury, calcium channel blockers are rarely mentioned. Most cases attributed to diltiazem have been marked by a short latency period (3 to 14 days) and features of hypersensitivity with fever, rash and eosinophilia. The pattern of liver injury was ranged from cholestatic to hepatocellular. Jaundice is often absent and usually mild. Autoantibody formation has not been described. Thus, liver injury from diltiazem is likely to be idiosyncratic in nature and is typically mild and self-limited with resolution within 4 to 8 weeks of stopping. Acute hepatic injury is listened as a possible adverse event in the diltiazem product label.
Likelihood score: C (probable but rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of diltiazem hepatotoxicity is not known, but most cases are probably due to hypersensitivity. Diltiazem is metabolized by the cytochrome P450 system and is an inhibitor of CYP 3A4 activity, which can lead to serious drug-drug interactions and potentiation of the hepatotoxic effects of other medications. Indeed, there have been several reports of clinically apparent liver injury or rhabdomyolysis occuring in patients on long term statins who had recently added diltiazem to their multidrug regimen, suggesting altered metabolism of the statin by the addition of a CYP 3A4 inhibitor.
Outcome and Management
Severity of liver injury from diltiazem ranges from mild and transient serum enzyme elevations to self-limited hepatitis with jaundice. Complete recovery is expected after stopping the drug and recovery is usually rapid (1 to 2 months). Diltiazem has not been implicated in cases of chronic liver injury or vanishing bile duct syndrome, but was the suspected agent in at least one published case of acute liver failure. Cross sensitivity of liver injury with other calcium channel blockers has been described, but not with diltiazem.
Drug Class: Cardiovascular Agents, Antihypertensive Agents, Calcium Channel Blockers
Other Drugs in the Subclass, Calcium Channel Blockers: Amlodipine, Felodipine, Isradipine, Nicardipine, Nifedipine, Nimodipine, Nisoldipine, Verapamil
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Diltiazem – Generic, Cardizem®
DRUG CLASS
Cardiovascular Agents
Product labeling at DailyMed, National Library of Medicine, NIH
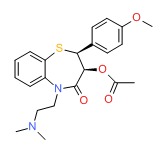
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Diltiazem | 42399-41-7 | C22-H26-N2-O4-S |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 11 January 2017
- Zimmerman HJ. Calcium channel blockers. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 646-7.(Expert review of hepatotoxicity published in 1999 mentions that among calcium channel blockers, diltiazem, nifedipine, bepridil and verapamil have been incriminated in instances of hepatic injury).
- De Marzio DH, Navarro VJ. Calcium channel blockers. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 524.(Review of hepatotoxicity of calcium channel blockers mentions that diltiazem and verapamil have been implicated in causing cholestatic liver injury in a small number of patients).
- Michel T, Hoffman BB. Calcium channel antagonists. Treatment of myocardial ischemia. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 755-60.(Textbook of pharmacology and therapeutics).
- McGraw BF. Diltiazem hydrochloride. Drug Intell Clin Pharm 1982; 16: 366-70. [PubMed: 7044735](Review of structure, chemistry, pharmacology, mechanism of action, clinical efficacy and safety of diltiazem; neither hepatotoxicity or ALT elevations are discussed in the section on adverse reactions).
- Sawai K. Effects of long-term administration of diltiazem hydrochloride in hypertensive patients. Clin Ther 1983; 5: 422-35. [PubMed: 6871924](Analysis of side effects in 8 patients with hypertension treated with diltiazem for up to 5 years; there were no significant changes in ALT or AST levels during treatment).
- Poulain D, Guimbail P, Florent F. [Median- and long-term tolerability of diltiazem]. Therapie 1984; 39: 177-84. French. [PubMed: 6729767](Among 162 patients with hypertension treated with diltiazem for 6-18 months, 3 [1.8%] had transient elevations in aminotransferase levels).
- Scolnick B, Brinberg D. Diltiazem and generalized lymphadenopathy. Ann Intern Med 1985; 102: 558. [PubMed: 3156549](50 year old man developed lymphadenopathy 4 days after starting diltiazem followed by rash and fever with normal liver tests initially which later rose [bilirubin 0.4 mg/dL, ALT 250 U/L, 6% eosinophils], symptoms resolving within a month of stopping).
- Sarachek NS, London RL, Matulewicz TJ. Diltiazem and granulomatous hepatitis. Gastroenterology 1985; 88: 1260-2. [PubMed: 3979751](54 year old man developed headache, fever and liver test elevations 2 weeks after starting diltiazem [bilirubin normal, ALT 254 U/L, Alk P 384 U/L, 3% eosinophils, liver biopsy showing granulomas], resolving within 3 weeks of stopping).
- Shallcross H, Padley SP, Glynn MJ, Gibbs DD. Fatal renal and hepatic toxicity after treatment with diltiazem. Br Med J 1987; 295:1236-7. [PMC free article: PMC1248306] [PubMed: 3120959](72 year old man developed rash followed by renal failure 2 days after switching from nifedipine to diltiazem, followed by jaundice and multiorgan failure [bilirubin not given, ALT 2500 U/L, Alk P not given] and death 1 week later).
- Buckley MM, Grant SM, Goa KL, McTavish D, Sorkin EM. Diltiazem. A reappraisal of its pharmacological properties and therapeutic use. Drugs 1990; 39: 757-806. [PubMed: 2191851](Review of pharmacology, clinical efficacy and safety of diltiazem; no mention of liver related side effects or effect on ALT levels).
- Toft E, Vyberg G, Therkelsen K. Diltiazem-induced granulomatous hepatitis. Histopathology 1991; 18: 474-5. [PubMed: 1885169](68 year old woman developed fever and fatigue 2 weeks after starting diltiazem [bilirubin not given, AST 94 U/L, Alk P 1331 U/L, liver biopsy showing granulomas], resolving within 5 weeks of stopping).
- Traverse JH, Swenson LJ, McBride JW. Acute hepatic injury after treatment with diltiazem. Am Heart J 1994; 127: 1636-9. [PubMed: 8197999](74 year old woman with angina developed sudden increase in liver tests [bilirubin 1.0 mg/dL, ALT 605 U/L, Alk P 60 U/L, LDH 990 U/L] 2 days after switching from nifedipine to diltiazem, with subsequent acidosis, hypoxia and hypotension, ultimately recovering and enzymes falling to normal within 10 days of stopping).
- Weir MR. Diltiazem: ten years of clinical experience in the treatment of hypertension. J Clin Pharmacol 1995; 35: 220-32. [PubMed: 7608309](Review of efficacy and safety of diltiazem in hypertension; adverse events leading to drug discontinuation occurred in 45 to 5% of patients taking diltiazem in randomized controlled trials; no details of liver related adverse events provided).
- Claas SA, Glasser SP. Long-acting diltiazem HCl for the chronotherapeutic treatment of hypertension and chronic stable angina pectoris. Expert Opin Pharmacother 2005; 6: 765-76. [PubMed: 15934903](Review of safety and efficacy of diltiazem; adverse events are reported to be no more frequent with diltiazem than with placebo; no hepatic adverse events were mentioned).
- Schroeder JS, Beier-Scott L, Ginsburg R, Bristow MR, McAuley BJ. Efficacy of diltiazem for medically refractory stable angina: long-term follow up. Clin Cardiol 1985; 8: 480-5. [PubMed: 2864152](Among 31 patients treated with diltiazem for 8 to 47 months, 5 had transient enzyme elevations and 3 had chronic asymptomatic elevations).
- Kanathur N, Mathai MG, Byrd RP Jr, Fields CL, Roy TM. Simvastatin-diltiazem drug interaction resulting in rhabdomyolysis and hepatitis. Tenn Med 2001; 94 (9): 339-41. [PubMed: 11550401](53 year old man developed nausea and fatigue 2 months after adding diltiazem to a chronic regimen of simvastatin [bilirubin 2.4 mg/dL, ALT 1707 U/L, Alk P 330 U/L, CPK 1390 U/L, myoglobin in the urine, protime 23 seconds], with resolution within 10 days of stopping both drugs).
- Lewin JJ 3rd, Nappi JM, Taylor MH. Rhabdomyolysis with concurrent atorvastatin and diltiazem. Ann Pharmacother 2002; 36: 1546-9. (60 year old man developed fatigue and abdominal pain 3 weeks after adding diltiazem to a drug regimen that included atorvastatin with rhabdomyosis and liver test abnormalities [bilirubin 0.7 mg/dL, ALT 1610 U/L, Alk P 287 U/L, CPK 1898 U/L] [PubMed: 12243603]that resolved within 3 months of stopping both drugs).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but none were attributed to a calcium channel blocker).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719](Summary of 25 years of adverse drug reaction reporting in Sweden identified 103 cases of drug induced acute liver failure; only one case was possibly linked to a calcium channel blocker--felodipine).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, calcium channel blockers were implicated as a sole agent in 2 cases [1 amlodipine, 1 verapamil] and as one of several agents in 2 cases [both amlodipine]).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, none of which were attributed to a calcium channel blocker).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to a calcium channel blocker or other antihypertensive medication).
- Deng W, Farricielli L. Hypoxic hepatitis and acute liver failure in a patient with newly onset atrial fibrillation and diltiazem infusion. BMJ Case Rep 2013; 2013. pii: bcr2013200573. [PMC free article: PMC3794224] [PubMed: 24042208](70 year old man with symptomatic atrial fibrillation developed hypotension 15 hours after starting intravenous diltiazem followed by transient severe liver injury [bilirubin 2.3 mg/dL, ALT 3472 U/L, Alk P not given, creatinine 4.4 mg/dL, and platelets 51,000/uL], resolving rapidly upon stopping suggestive of ischemic hepatitis).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases; one case was attributed to verapamil, but none were linked to diltiazem or other calcium channel blockers).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 39 [4%] were due to antihypertensive agents including 4 due to calcium channel blockers [amlodipine in 1 and verapamil in 3 instances], but none to diltiazem).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Calcium Channel Blockers.[LiverTox: Clinical and Researc...]Review Calcium Channel Blockers.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Verapamil.[LiverTox: Clinical and Researc...]Review Verapamil.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Amlodipine.[LiverTox: Clinical and Researc...]Review Amlodipine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Nifedipine.[LiverTox: Clinical and Researc...]Review Nifedipine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Nicardipine.[LiverTox: Clinical and Researc...]Review Nicardipine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Diltiazem - LiverToxDiltiazem - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...