NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Caffeine is xanthine alkaloid that occurs naturally in seeds, leaves and fruit of several plants and trees that acts as a natural pesticide. Caffeine is a major component of coffee, tea and chocolate and in humans acts as a central nervous system (CNS) stimulant. Consumption of caffeine, even in high doses, has not been associated with elevations in serum enzyme elevations or instances of clinically apparent liver injury.
Background
Caffeine is a psychoactive xanthine alkaloid that is a major component of coffee, tea and some foods (chocolate) and is the most frequently used psychoactive agent. At least 90% of adults in the United States consume caffeine daily. Caffeine is also found in several herbs including yerba mate, green coffee beans, guarana berries and yaupon holly. Caffeine is a frequent component of multiingredient dietary supplements, particularly those used for weight loss, improving athletic performance, increasing alertness and decreasing sleepiness. Finally, caffeine is available over-the-counter in tablets generally of 100 or 200 mg for improving alertness and decreasing sleepiness. Caffeine acts as a CNS stimulant, probably by displacing adenosine from A1 or A2 receptors in the brain. Adenosine in the circulation suppresses neural activity and is believed to be responsible for the feeling of sleepiness via activation of sleep promoting neurons. Reversal of the actions of adenosine may account for the psychoactive characteristics of caffeine containing products. Caffeine has many other actions including inhibition of other neurotransmitters as well as antiinflammatory and immunomodulatory actions. Nevertheless, caffeine is not formally approved as a medication for any condition or disorder. Caffeine is, however, the most commonly used psychoactive drug in the world, largely because of its presence in coffee and tea. The concentration of caffeine in coffee varies greatly by method of preparation and source, but typically ranges from 80 to 175 mg per cup of coffee, and from 20 to 80 mg per cup of tea. The amount in chocolate generally ranges from 10 to 30 mg per bar. Soft drinks also contain caffeine usually in amounts similar to tea. Recently, energy drinks have become popular (Red Bull, Jolt, Monster, and others) which may contain added caffeine in concentrations of 80 to more than 200 mg per can or bottle. Caffeine is a frequent component of multiingredient dietary supplements which may not provide the actual concentrations but are often in the range of 50 to 250 mg per serving. Caffeine in typical amounts is well tolerated and not associated with long term adverse outcomes. In high doses however, generally in excess of 1000 mg daily, caffeine can be toxic. Symptoms include nervousness, irritability, insomnia, headaches, rapid heartbeat, tremor, and gastrointestinal discomfort. Severe toxicity is marked by confusion, excessive anxiety, mania, hallucinations, seizures, rhabdomyolysis, cardiorespiratory arrest and death.
Hepatotoxicity
Some degree of caffeine intake is almost universal in modern society and an estimated 90% of adults in the United States consume caffeine daily, the average amount being 200 mg daily. Yet despite its widescale use, there is no evidence that regular consumption of caffeine or coffee has adverse effects on the liver. Indeed, epidemiological studies suggest that regular coffee intake may have modest protective effects against the progression of chronic liver disease and development of liver cancer. In high, toxic doses, caffeine can have severe effects on brain, heart and muscle function but has not been linked to clinically apparent liver injury. In contrast, there have been several reports of liver injury linked to use of caffeine rich energy drinks. These reports have not been very convincing and most were not well documented. In many instances, the hepatic injury resembled acute hepatic necrosis or ischemic hepatitis (Case 1). In other cases, other diagnoses were more likely than liver injury from the energy drinks (Case 2). Furthermore, it remains unclear whether the hepatic effects were caused by caffeine per se or to other components in typical energy drinks, such as vitamins, herbs or other botanical products. In reports of caffeine overdose including cases with autopsies, hepatic injury has been absent or not mentioned. Thus, caffeine is unlikely to cause liver injury, but the various high caffeine energy drinks which are widely used may possibly cause liver injury when used to excess.
Likelihood score for caffeine: E (unlikely cause of clinically apparent liver injury).
Likelihood score for energy drinks: C[H] (probable rare cause of clinically apparent liver injury when used in high amounts).
Mechanism of Injury
Caffeine is metabolized by the microsomal P450 drug metabolizing enzymes, predominantly CYP 1A2. Patients with advanced cirrhosis may have delayed metabolism of caffeine and experience caffeine side effects (nervousness, insomnia, headache) at levels of intake that are well tolerated by patients without liver disease.
Energy drinks typically have high concentrations of caffeine but also a myriad of other components including vitamins, minerals, amino acids, sugar and various herbal products, the concentration and purity of which are usually unknown.
Drug Class: CNS Stimulants, Xanthine Derivatives
Other Drugs in the Class: Theophylline
See also Energy Drinks
CASE REPORT
Case 1. Acute liver injury attributed to use of energy drinks.(1)
A 22 year old woman developed low grade fever, abdominal pain, nausea and vomiting and was found to have abnormal liver tests having been consuming 10 cans of energy drinks daily for 2 weeks. On examination in the emergency room, she had epigastric tenderness but no other findings and was sent home. The following day she developed jaundice and was seen again and admitted to the hospital. She denied alcohol or drug abuse and was not taking other medications. Laboratory tests showed marked elevations in ALT and AST (Table) but normal levels of bilirubin, alkaline phosphatase and GGT. The INR was 1.6. Acetaminophen levels were undetectable. There was no serologic evidence of acute hepatis A, B, C or E and tests for Epstein Barr virus and cytomegalovirus infection were negative. She was managed conservatively. She did not undergo liver biopsy or imaging studies. Serum aminotransferase levels decreased rapidly and she was discharged four days later. In follow up one month later, she was asymptomatic and serum ALT levels were normal.
Key Points
| Medication: | Energy drinks (10 cans daily for 2 weeks) |
|---|---|
| Pattern: | Hepatocellular (R=~172) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 2 weeks |
| Recovery: | Within 1 month |
| Other medications: | None |
Laboratory Values
Comment
This short report described a young woman who presented with acute hepatic necrosis that was attributed to consumption of energy drinks for two weeks. The marked ALT and AST levels, the R ratio above 100, the abnormality of INR with minimal elevation in bilirubin and the rapid improvement within a week of onset are typical features of acute hepatic necrosis and not typical at all of the acute hepatitis seen with idiosyncratic drug induced liver injury. The clinical presentation was similar to that of acute acetaminophen overdose or acute ischemic hepatitis, both of which might be considered more likely than acute injury from a commercial energy drink. The components of the energy drink that were responsible for the severe acute liver injury were not clear. The product name of the energy drink was not provided but the ingredients listed in the report included vitamins, minerals and an “energy blend” which was likely to include caffeine, but other herbal components were not given.
Case 2. Acute hepatitis attributed to use of energy drinks.(2)
A 50 year old previously healthy man developed nausea, anorexia, epigastric pain and fatigue followed by dark urine and jaundice and was found to have markedly abnormal liver tests. He reported that he had been drinking 4 to 5 cans of an energy drink for the previous 3 weeks. He denied a history of liver disease, drug allergies, alcohol abuse, injection drug use or risk factors for viral hepatitis. He was not taking other medications or over-the-counter products. Initially, total serum bilirubin was 10.3 mg/dL (direct 7.7), ALT 1203 U/L, AST 1802 U/L, alkaline phosphatase 206 U/L, and INR 1.0. Tests for acute hepatitis A, B and E were negative, but he was positive for both anti-HCV and HCV RNA (5.6 million IU/mL). Antinuclear and smooth muscle antibodies were negative. Ultrasound of the abdomen showed no gallstones or evidence of biliary obstruction. A liver biopsy showed acute hepatitis with bridging necrosis and cholestasis but no fibrosis. Despite the HCV serology, he was diagnosed as having liver injury due to consumption of energy drinks, the suspected ingredient being niacin. After worsening for a few days, he began to improve spontaneously (Table) and in follow up serum weeks later he was asymptomatic and serum aminotransferase levels were normal. Follow up HCV RNA results were not reported.
Key Points
| Medication: | Energy drinks (4-5 cans daily for 3 weeks) |
|---|---|
| Pattern: | Hepatocellular (R=~18.8) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 3 weeks |
| Recovery: | Unclear in timing |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | AST (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|---|
| 3 weeks | 1 day | 1203 | 1802 | 206 | 10.3 | Hospitalized |
| 2 days | 1112 | 1710 | 11.5 | Anti-HCV and HCV RNA positive | ||
| 3 days | 1368 | 2435 | 13.4 | Liver biopsy | ||
| 4 days | 2073 | 4051 | 19.3 | |||
| 5 days | 1629 | 2674 | 15.7 | |||
| 6 days | 1224 | 1695 | 12.0 | Discharged, asymptomatic | ||
| 5 weeks | 14 days | 90 | 35 | 2.7 | ||
| 18 days | 23 | 36 | 2.2 | |||
| Normal Values | <38 | <42 | <130 | <1.2 | ||
Comment
This case report described a 50 year old construction worker who presented with acute hepatitis three weeks after starting regular consumption of energy drinks (4 to 5 cans of an undeclared product daily). Importantly, he also tested positive for anti-HCV and HCV RNA despite having no risk factors for hepatitis C and no previous knowledge of being infected. The authors attributed the acute hepatitis to consumption of the energy drinks and not to hepatitis C, arguing that he more likely had an asymptomatic chronic HCV infection. However, the clinical presentation, liver biopsy findings and subsequent course are entirely compatible with acute hepatitis C. The challenge in this diagnosis is that serologic tests (including IgM anti-HCV) are unreliable in separating acute from chronic infection, and definitive diagnosis acute vs chronic HCV infection depends upon documentation of seroconversion to anti-HCV positivity. The diagnosis can also be fairly reliably made if the infection resolves as the hepatitis resolves, with disappearance of HCV RNA (and rising titers of anti-HCV) or if there is a recent clear history of exposure. Such documentation, however, is not always available and a high proportion of patients with acute hepatitis C develop de novo chronic infection. In large case series, acute hepatitis C accounts for 1 to 5% of cases initially suspected to be drug induced liver injury. Of the six reported cases of acute liver injury from energy drink consumption published between 2000 and 2020, none were very convincing and most had other possible explanations (ischemic hepatitis, liver injury from other drugs being taken, acetaminophen or other toxic overdose).
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Caffeine – Generic, NoDoz®
DRUG CLASS
CNS Stimulants, Xanthine Derivatives
Product labeling at DailyMed, National Library of Medicine, NIH
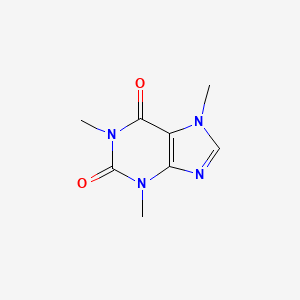
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Caffeine | 58-08-2 | C8-H10-N4-O2 |
|
CITED REFERENCES
- 1.
- Vivekanandarajah A, Ni S, Waked A. Acute hepatitis in a woman following excessive ingestion of an energy drink: a case report. J Med Case Rep. 2011 Jun 22;5:227. [PMC free article: PMC3141691] [PubMed: 21696583]
- 2.
- Harb JN, Taylor ZA, Khullar V, Sattari M. Rare cause of acute hepatitis: a common energy drink. BMJ Case Rep. 2016;2016:bcr2016216612. [PMC free article: PMC5129143] [PubMed: 27803015]
ANNOTATED BIBLIOGRAPHY
References updated: 18 June 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999; caffeine is not discussed).
- O’Brien CP. Caffeine. In, Drug use disorders and addiction. In, Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 440.(Textbook of pharmacology and therapeutics).
- Benowitz NL, Osterloh J, Goldschlager N, Kaysen G, Pond S, Forhan S. Massive catecholamine release from caffeine poisoning. JAMA. 1982;248:1097–8. [PubMed: 7109204](30 year old woman took an overdose of caffeine [NoDoz 240 tablets: 24 gm] and developed confusion, emesis, tachycardia, metabolic acidosis and hyperglycemia within hours, resolving with fluid replacement and support within 3 days; liver tests showed “no abnormalities”).
- Winek CL, Wahba W, Williams K, Blenko J, Janssen J. Caffeine fatality: a case report. Forensic Sci Int. 1985;29(3-4):207–11. [PubMed: 4076952](21 year old woman took an overdose of 70 tablets that she thought were amobarbital but actually were mostly caffeine and had a fatal cardiorespiratory arrest, autopsy demonstrating caffeine blood levels of 240 mg/L).
- Zimmerman PM, Pulliam J, Schwengels J, MacDonald SE. Caffeine intoxication: a near fatality. Ann Emerg Med. 1985;14:1227–9. [PubMed: 4061999](37 year old woman took an overdose of 27 gm of caffeine and rapidly developed hypotension, tachycardia, confusion, acidosis and coma [pH 7.2] followed by repeated episodes of ventricular fibrillation, treated successfully with dialysis and medical support [initial caffeine level 199 mg/L] allowing for discharge 27 days later).
- Cannon ME, Cooke CT, McCarthy JS. Caffeine-induced cardiac arrhythmia: an unrecognised danger of healthfood products. Med J Aust. 2001;174:520–1. [PubMed: 11419773](25 year old woman with known mitral value prolapse had a fatal, witnessed cardiac arrest [ventricular fibrillation] having just consumed a large bottle of an energy drink [Race 2005: 300-570 mg of caffeine]; no mention of hepatic abnormalities).
- Holmgren P, Nordén-Pettersson L, Ahlner J. Caffeine fatalities--four case reports. Forensic Sci Int. 2004;139:71–3. [PubMed: 14687776](4 patients with fatal caffeine overdoses with lethal levels of caffeine in postmortem blood tests; no description of hepatic effects, although three had known risk factors for liver disease [alcoholism, drug abuse, hepatitis C]).
- Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24–32. [PubMed: 15633120](Among 5944 adults evaluated in the US NHANES population based study who had risk factors for liver disease, 8.7% had raised serum ALT levels and increasing levels of coffee consumption and caffeine intake were associated with lower ALT levels suggesting that coffee intake may mitigate liver injury).
- Ruhl CE, Everhart JE. Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. Gastroenterology. 2005;129:1928–36. [PubMed: 16344061](Among 9849 US adults interviewed in 1982-4 and followed in the US NHANES population based study, the risk of death or hospitalization for chronic liver disease was 1.4% overall at 20 years, but was lower in those who drank at least 2 cups of coffee or tea daily [1.1%], vs 1-2 cups [1.6%] or less than 1 cup [1.8%]).
- Kerrigan S, Lindsey T. Fatal caffeine overdose: two case reports. Forensic Sci Int. 2005;153:67–9. [PubMed: 15935584](Two patients with fatal caffeine overdose, 39 year old woman with known drug use found dead and 29 year old male with obesity, diabetes and known overdose with caffeine pills and vomiting and seizures, had postmortem blood caffeine levels of 192 and 567 mg/L; no mention of liver abnormalities).
- Iyadurai SJ, Chung SS. New-onset seizures in adults: possible association with consumption of popular energy drinks. Epilepsy Behav. 2007;10:504–8. [PubMed: 17349826](Four adults had new onset seizures accompanied by transient tachycardia and hypertension shortly after consuming energy drinks [Rockstar, Monster], usually on an empty stomach or with diet pills and not recurring during follow up when the energy drinks were stopped; all four had no “laboratory abnormalities” except for high glucose in one).
- Muncie HL. The safety of caffeine consumption. Am Fam Physician. 2007;76:1282–1285-6. [PubMed: 18019871](Discussion of the safety of caffeine in regards to cardiovascular disease, diabetes, renal disease and cancer concludes that patients can safely consume 2-3 cups of coffee or the equivalent amount of caffeine daily).
- Haller C, Kearney T, Bent S, Ko R, Benowitz N, Olson K. Dietary supplement adverse events: report of a one-year poison center surveillance project. J Med Toxicol. 2008;4:84–92. [PMC free article: PMC3550135] [PubMed: 18570167](Among 275 poison control center calls about dietary supplements occurring over a 12 month period, 112 [41%] were symptomatic, 8 resulted in hospitalization and one was fatal, the majority of symptomatic cases [47%] were related to a caffeine containing multiingredient products; no mention of hepatotoxicity).
- Berger AJ, Alford K. Cardiac arrest in a young man following excess consumption of caffeinated "energy drinks". Med J Aust. 2009;190:41–3. [PubMed: 19120009](28 year old man had cardiac arrest [ventricular fibrillation] shortly after consuming 7-8 cans of an energy drink [~640 mg of caffeine] and underwent extensive evaluation including coronary angiography which was normal; no mention of liver test abnormalities).
- Higgins JP, Tuttle TD, Higgins CL. Energy beverages: content and safety. Mayo Clin Proc. 2010;85:1033–41. [PMC free article: PMC2966367] [PubMed: 21037046](Review of the increasing popularity of energy drinks which often have high levels of caffeine [50-505 mg/serving], but are not regulated in regard to caffeine concentration or claims for performance enhancement; other ingredients may include glucose, taurine, branched chain amino acids, various vitamins, ginseng, guarana, ginkgo, milk thistle, L-carnitine and citric acid).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to caffeine or energy drinks).
- Pelchovitz DJ, Goldberger JJ. Caffeine and cardiac arrhythmias: a review of the evidence. Am J Med. 2011;124:284–9. [PubMed: 21435415](Caffeine is a nonselective competitive antagonist of adenosine receptor subtypes A1 and A2A, which in concentrations typically consumed by adults and chronic use [up to 4 cups of coffee daily], has not been associated with excess cardiovascular mortality; there is little evidence that regular use of caffeine is associated with an increased risk of arrhythmias even in high risk subjects).
- Vivekanandarajah A, Ni S, Waked A. Acute hepatitis in a woman following excessive ingestion of an energy drink: a case report. J Med Case Rep. 2011 Jun 22;5:227. [PMC free article: PMC3141691] [PubMed: 21696583](22 year old woman developed abdominal pain, nausea and vomiting having consumed 10 cans of energy drinks daily for 2 weeks [bilirubin 1.7 rising to 3.5 mg/dL, ALT 216 rising to 7553 U/L, Alk P and GGT normal, INR 1.6], recovering within the next few weeks with no specific therapy: Case 1).
- Apestegui CA, Julliard O, Ciccarelli O, Duc DK, Lerut J. Energy drinks: another red flag for the liver allograft. Liver Transpl. 2011;17:1117–8. [PubMed: 21674755](16 year old man with liver transplant developed sudden rise in liver tests on two occasions one year apart after consuming 3-5 cans of Red Bull daily [ALT 6 and 26 times ULN, bilirubin peak of 2.3 and 10.7 mg/dL], resolving in 4-8 months).
- Trabulo D, Marques S, Pedroso E. Caffeinated energy drink intoxication. BMJ Case Rep. 2011;2011:bcr0920103322. [PMC free article: PMC3062360] [PubMed: 22714613](28 year old man had sudden onset of seizures followed by lactic acidosis, coma and respiratory arrest shortly after drinking several cans of an energy drink [Red Bull] together with coffee, recovering within a week with medical support; no mention of liver test abnormalities although he had a history of injection drug use and hepatitis C).
- Babu KM, Zuckerman MD, Cherkes JK, Hack JB. First-onset seizure after use of an energy drink. Pediatr Emerg Care. 2011;27:539–40. [PubMed: 21642791](15 year old adolescent boy developed seizures after drinking 2 bottles of “5-hour ENERGY” with emesis and tachycardia [caffeine level 99 mg/L]; he had no further seizures in follow up without anticonvulsant therapy).
- Calabrò RS, Italiano D, Gervasi G, Bramanti P. Single tonic-clonic seizure after energy drink abuse. Epilepsy Behav. 2012;23:384–5. [PubMed: 22370117](20 year old man had a single seizure having consumed 4-6 cans of Red Bull daily for 5 months and had no further seizures in follow up after stopping energy drink consumption).
- Wolk BJ, Ganetsky M, Babu KM. Toxicity of energy drinks. Curr Opin Pediatr. 2012;24:243–51. [PubMed: 22426157](Review of the literature on adverse events linked to consumption of energy drinks including cardiovascular [arrhythmias, sudden death], renal [acute renal failure], hepatic [acute hepatitis], neurologic [seizures] and psychiatric effects [anxiety, poor sleep, developmental problems], the ingredients responsible for the adverse events not always being attributable to caffeine).
- Sepkowitz KA. Energy drinks and caffeine-related adverse effects. JAMA. 2013;309:243–4. [PubMed: 23330171](History of FDA actions in limiting and regulating caffeine containing products, including warning letters concerning energy drinks, mentions that many products do not list the concentration of caffeine and that there may be great individual variation in sensitivity or pharmacodynamics of caffeine which can be affected by underlying liver disease, alcohol use and medications that are metabolized via CYP 1A2).
- Noff T, Insel J. Energy drinks and the unwanted buzz: a case report. Md Med. 2013;13:28–9. [PubMed: 23556369](46 year old woman with coronary artery disease developed severe chest pain having consumed 3-5 energy drinks daily for 6 months and was found to have abnormal serum aminotransferase levels [bilirubin normal, ALT 325 U/L, AST 437 U/L], which the authors attributed to niacin [30 mg per serving] in the energy drinks; no further details provided).
- Consumption of Caffeine in Food and Dietary Supplements; Food and Nutrition Board; Board on Health Sciences Policy; Institute of Medicine. Caffeine in Food and Dietary Supplements: Examining Safety: Workshop Summary. Washington (DC): National Academies Press (US); 2014 Apr 23. [PubMed: 24872990](Summary of a workshop on the consumption of caffeine in the United States and evidence for and against cardiovascular and central nervous system safety; no discussion of liver toxicity).
- Huang B, Kunkel D, Kabany ME. Acute liver failure following one year of daily consumption of a sugar-free energy drink. ACG Case Rep J. 2014;1(4):214–6. [PMC free article: PMC4435335] [PubMed: 26157880](36 year old man developed jaundice having consumed 3 energy drinks [Rockstar] daily for a year and having a 15 year history of binge alcohol drinking [bilirubin 16.1 mg/dL, ALT 2995 U/L, Alk P 231 U/L, INR 1.0], with subsequent worsening [bilirubin rising to 23.1 mg/dL, INR 3.7, hepatic encephalopathy] leading to successful liver transplantation).
- Gurley BJ, Steelman SC, Thomas SL. Multi-ingredient, caffeine-containing dietary supplements: history, safety, and efficacy. Clin Ther. 2015;37:275–301. [PubMed: 25262198](Extensive review of the history, clinical efficacy and safety of multiingredient, caffeine containing dietary supplements which have been widely used as stimulants and weight loss agents and may have greater cardiovascular and neurologic adverse effects because of increase in caffeine toxicity caused by interaction with other components in the supplements such as taurine, p-synephrine, yohimbine, green tea, theanine, dimethylamylamine [DMAA], diethylphenylethylamine [PEA] and, previously, ephedra).
- Eichner ER. Fatal caffeine overdose and other risks from dietary supplements. Curr Sports Med Rep. 2014;13:353–4. [PubMed: 25391087](Editorial on the dangers including fatalities of unregulated dietary supplements that may contain excessive amounts of caffeine or have toxicities due to contaminants such as amphetamines [Frenzy, Craze] or aegeline [OxyELITE Pro]).
- Dickson JC, Liese AD, Lorenzo C, Haffner SM, Watkins SM, Hamren SJ, Stiles JK, et al. Associations of coffee consumption with markers of liver injury in the insulin resistance atherosclerosis study. BMC Gastroenterol. 2015;15:88. [PMC free article: PMC4515880] [PubMed: 26215323](Among 1005 nondiabetic patients enrolled in a study of insulin resistance and cardiovascular risk, ALT and AST levels were similar across different average daily coffee intake groups, but were lower in patients with higher intakes of coffee in multivariate analyses controlling for age, sex and ethnicity).
- Doepker C, Lieberman HR, Smith AP, Peck JD, El-Sohemy A, Welsh BT. Caffeine: friend or foe? Annu Rev Food Sci Technol. 2016;7:117–37. [PubMed: 26735800](Expert panel review of the risks and benefits of caffeine focusing largely on cardiovascular, reproductive health and behavioral effects).
- Harb JN, Taylor ZA, Khullar V, Sattari M. Rare cause of acute hepatitis: a common energy drink. BMJ Case Rep. 2016;2016:bcr2016216612. [PMC free article: PMC5129143] [PubMed: 27803015](50 year old man developed an acute hepatitis 3 weeks after consuming 4-5 energy drinks daily [bilirubin 10.3 rising to 19.3 mg/dL, ALT 1203 U/L, Alk P 206 U/L, anti-HCV and HCV RNA positive], with rapid resolution on stopping the energy drinks but no information on HCV RNA levels in follow up).
- Temple JL, Bernard C, Lipshultz SE, Czachor JD, Westphal JA, Mestre MA. The safety of ingested caffeine: a comprehensive review. Front Psychiatry. 2017;8:80. [PMC free article: PMC5445139] [PubMed: 28603504](Review of the safety of caffeine for healthy as well as vulnerable populations concludes that daily intake of up to 400 mg of caffeine is probably safe for healthy adults, but lower levels should be recommended for children and pregnant women).
- Higgins JP, Babu K, Deuster PA, Shearer J. Energy drinks: a contemporary issues paper. Curr Sports Med Rep. 2018;17:65–72. [PubMed: 29420350](Review of the definition, efficacy and safety of energy drinks which have become increasingly popular, particular among high school and college students, athletes and body builders, and are defined as high caffeine containing beverages that usually contain miscellaneous other components such as vitamins, minerals, amino acids, and herbal products such as ginseng, yohimbe and ginkgo; adverse effects are largely attributable to caffeine and include neurologic and cardiovascular effects, with only rare, isolated reports of liver or kidney injury).
- Harty PS, Zabriskie HA, Erickson JL, Molling PE, Kerksick CM, Jagim AR. Multi-ingredient pre-workout supplements, safety implications, and performance outcomes: a brief review. J Int Soc Sports Nutr. 2018;15:41. [PMC free article: PMC6083567] [PubMed: 30089501](Review of the efficacy and safety of multiingredient pre-workout supplements which typically contain caffeine, amino acids, nitrates and creatine and are “relatively safe with minimal reported adverse events”, mentions that their use has not been linked to serum enzyme elevations; no discussion of hepatotoxicity or concurrent use of anabolic steroids).
- Al Yacoub R, Luczkiewicz D, Kerr C. Acute kidney injury and hepatitis associated with energy drink consumption: a case report. J Med Case Rep. 2020;14:23. [PMC free article: PMC6988357] [PubMed: 31992329](62 year old woman with advanced small cell lung cancer in hospice developed nausea, vomiting and confusion and was found to have abnormal liver tests [bilirubin 0.3 mg/dL, ALT 2866 U/L, AST 4333 U/L, Alk P 111 U/L], having had little oral intake for 2 weeks except for 5-6 cans of an energy drink daily).
- van Dam RM, Hu FB, Willett WC. Coffee, caffeine, and health. N Engl J Med. 2020;383:369–78. [PubMed: 32706535](Review of the metabolism, physiologic and toxic effects of caffeine and coffee consumption including discussion of possible beneficial effects of coffee in chronic liver disease).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Teratogen update: evaluation of the reproductive and developmental risks of caffeine.[Teratology. 2001]Review Teratogen update: evaluation of the reproductive and developmental risks of caffeine.Christian MS, Brent RL. Teratology. 2001 Jul; 64(1):51-78.
- Review Caffeine and Its Antioxidant Properties-It Is All about Dose and Source.[Int J Mol Sci. 2022]Review Caffeine and Its Antioxidant Properties-It Is All about Dose and Source.Ősz BE, Jîtcă G, Ștefănescu RE, Pușcaș A, Tero-Vescan A, Vari CE. Int J Mol Sci. 2022 Oct 28; 23(21). Epub 2022 Oct 28.
- Autopsy report for a caffeine intoxication case and review of the current literature.[J Toxicol Pathol. 2015]Autopsy report for a caffeine intoxication case and review of the current literature.Yamamoto T, Yoshizawa K, Kubo S, Emoto Y, Hara K, Waters B, Umehara T, Murase T, Ikematsu K. J Toxicol Pathol. 2015 Jan; 28(1):33-6. Epub 2014 Dec 7.
- Coffee and Caffeine Consumption for Human Health.[Nutrients. 2021]Coffee and Caffeine Consumption for Human Health.Abalo R. Nutrients. 2021 Aug 24; 13(9). Epub 2021 Aug 24.
- Molecular and biochemical characterization of caffeine synthase and purine alkaloid concentration in guarana fruit.[Phytochemistry. 2014]Molecular and biochemical characterization of caffeine synthase and purine alkaloid concentration in guarana fruit.Schimpl FC, Kiyota E, Mayer JL, Gonçalves JF, da Silva JF, Mazzafera P. Phytochemistry. 2014 Sep; 105:25-36. Epub 2014 May 21.
- Caffeine - LiverToxCaffeine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...