NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Energy drinks are defined as over-the-counter commercial beverages with high caffeine content that are advertised as boosting energy including mental alertness and physical performance. More than 50 brands of energy drinks are available in grocery stores, nutrition centers, beverage shops and on the internet. Consumption of excess energy drinks has been linked to several instances of clinically apparent acute liver injury which can be severe and result in fatal or urgent liver transplantation. The components in energy drinks that account for the liver injury are not clear and caffeine by itself has not been linked to hepatic injury.
Background
Energy drinks are defined as beverages with high concentrations of caffeine that are purported to boost energy, physical and athletic performance and mental alertness. Energy drinks were first marketed in the late 1990s and have subsequently become popular and widely available. The commercial products vary greatly in concentration of caffeine as well as in other characteristics, such as carbonation, flavoring, sugar and sweeteners, vitamins, minerals, amino acids and botanical ingredients. Popular brands include Red Bull, Monster, Rockstar, NOS Energy, Xyience, Bang, Celsius, Zevia, Full Throttle and Kick Start. The caffeine content of these products varies from 5 to 40 mg per ounce, and a single serving (8 to 24 ounces; 235-710 mL) may contain up to 300 mg of caffeine. Caffeine is also present in regular soft drinks but generally in amounts similar to tea (2 to 5 mg/oz; and 24 to 60 mg per 12 oz can), although soft drink brands with extra caffeine have also become popular (Pepsi Max, Jolt Cola, Mountain Dew Energy). The average caffeine content in selected energy drinks and soft drinks is shown in the table below. Caffeine has been shown to have psychoactive properties and to increase alertness and wakefulness and improve physical and athletic performance, although to a minor degree and with considerable inter-individual variability. Caffeine in typical amounts is well tolerated and not associated with significant side effects or long term adverse outcomes. Adverse side effects of caffeine are generally dose related and can include nervousness, irritability, insomnia, headaches, rapid heartbeat, tremor, and gastrointestinal discomfort. In high doses, generally in excess of 1000 mg daily, caffeine can have severe toxicity, marked by confusion, excessive anxiety, mania, hallucinations, seizures, rhabdomyolysis, cardiorespiratory arrest and death.
Caffeine Content of Selected Soft Drinks and Energy Drinks Available in the United States
| Product Name | Caffeine (mg/oz) | Typical size (Can/Bottle) | Caffeine (mg/Serving) | Comments, Selected Other Ingredients |
|---|---|---|---|---|
| Soft Drinks | ||||
| Coca-Cola | 2.9 | 12 oz | 23 | |
| Pepsi | 3.2 | 12 oz | 38 | |
| Dr Pepper | 3.4 | 12 oz | 41 | |
| Mountain Dew | 4.5 | 12 oz | 54 | |
| Diet Pepsi | 2.9 | 12 oz | 35 | |
| Diet Dr Pepper | 3.4 | 12 oz | 41 | |
| Diet Coke | 3.8 | 12 oz | 46 | |
| Diet Mountain Dew | 4.5 | 12 oz | 54 | |
| Sprite | 0 | 12 oz | 0 | |
| 7-Up | 0 | 12 oz | 0 | |
| Fortified Soft Drinks | ||||
| Pepsi Max | 3.8 | 8 oz | 43 | |
| Mountain Dew Kickstart | 5.8 | 16 oz | 92 | |
| Coca-Cola Energy | 9.5 | 12 oz | 114 | |
| AMP Energy | 9 | 16 oz | 142 | |
| Energy Drinks | ||||
| Red Bull | 10 | 8 oz | 80 | Most commonly used energy drink |
| V8 Fusion | 10 | 8 oz | 80 | 50% vegetable juice |
| Zevia | 10 | 12 oz | 120 | Stevia as sweetener, calorie-less |
| Rockstar | 10 | 16 oz | 160 | Taurine, ginseng, ginkgo, milk thistle |
| NOS | 10 | 16 oz | 160 | Taurine, acacia, B vit |
| Jolt Cola | 10 | 16 oz | 160 | |
| Xyience | 11 | 16 oz | 176 | Ginseng, Guarana, vit, aa, min |
| Celsius | 12 | 16 oz | 200 | Green tea, guarana, ginger, vit, min |
| 5-Hour Energy | 100 | 2 oz | 200 | Vit, aa |
| Spike Shooter | 36 | 8 oz | 300 | Yohimbine, vit, aa “Spike S formula” |
| Redline Xtreme | 40 | 8 oz | 316 | Green tea, yohimbe, yerba mate, aa |
| Other | ||||
| Typical Drip Coffee | 8-20 | 1 cup, 8 oz | 60-150 | |
| Typical Black Tea | 2 to 5 | 1 cup, 8 oz | 15-35 | |
- *
Concentrations and volumes are rounded off. Several of the commercial products have multiple formulations, flavors and special names. Abbreviations: aa, amino acids; min, minerals; vit, vitamins.
Hepatotoxicity
Caffeine containing energy drinks are widely used and generally well tolerated. Neverless, when taken in excessive amounts they can lead to caffeine toxicity with tremors, confusion, mania, stupor and coma and cardiac arrhythmias and cardiorespiratory failure. In addition, there have been several single case reports of liver injury linked to use of caffeine-rich energy drinks. These reports were often incompletely documented and not completely convincing. In several instances, the hepatic injury resembled acute hepatic necrosis or ischemic hepatitis (Case 1) that may have been due to a drug overdose or cardiac arrest. In other cases, other diagnoses were not completely ruled out (Case 2). Furthermore, it remains unclear whether the hepatic effects of the energy drinks were caused by caffeine per se or to other components included in their formulation, such as vitamins, herbs or other botanical products. The reports of caffeine overdose including cases with autopsies, hepatic injury has been absent or not mentioned. Thus, caffeine is unlikely to cause liver injury, but the various high caffeine energy drinks which are widely used may possibly cause liver injury when used to excess.
Likelihood score: C[H] (probable rare cause of clinically apparent liver injury when used in high amounts).
Mechanism of Injury
Caffeine is metabolized by the microsomal P450 drug metabolizing enzymes, predominantly CYP 1A2. Patients with advanced cirrhosis may have delayed metabolism of caffeine and experience caffeine side effects (nervousness, insomnia, headache) at levels of intake that are well tolerated by patients without liver disease.
Energy drinks typically have high concentrations of caffeine but also a myriad of other components including vitamins, minerals, amino acids, sugar and various herbal products, the concentration and purity of which are usually unknown.
Drug Class: CNS Stimulants, Xanthine Derivatives
See also Caffeine
CASE REPORT
Case 1. Acute liver injury attributed to use of energy drinks.(1)
A 22 year old woman developed low grade fever, abdominal pain, nausea and vomiting and was found to have abnormal liver tests after having consumed 10 cans of energy drinks daily for 2 weeks. On examination in the emergency room, she had epigastric tenderness but no other findings and was sent home. The following day she developed jaundice and was admitted to the hospital. She denied alcohol or drug abuse and was not taking other medications. Laboratory tests showed marked elevations in ALT and AST (Table) but normal levels of bilirubin, alkaline phosphatase and GGT. The INR was 1.6. Acetaminophen levels were undetectable. There was no serologic evidence of acute hepatis A, B, C or E and tests for Epstein Barr virus and cytomegalovirus infection were negative. She was managed conservatively. She did not undergo liver biopsy or imaging studies. Serum aminotransferase levels decreased rapidly and she was discharged four days later. In follow up one month later, she was asymptomatic and serum ALT levels were normal.
Key Points
| Medication: | Energy drinks (10 cans daily for 2 weeks) |
|---|---|
| Pattern: | Hepatocellular (R=~172) |
| Severity: | 3+ (jaundice and Hospitalization) |
| Latency: | 2 weeks |
| Recovery: | Within 1 month |
| Other medications: | None |
Laboratory Values
Comment
This short report described a young woman who presented with acute hepatic necrosis that was attributed to consumption of energy drinks for two weeks. The marked ALT and AST levels, the R ratio above 100, the abnormality of INR with minimal elevation in bilirubin and the rapid improvement within a week of onset are typical features of acute hepatic necrosis and not typical of the acute hepatitis seen with idiosyncratic drug induced liver injury. The clinical presentation was similar to that of acute acetaminophen overdose or acute ischemic hepatitis, both of which might be considered more likely than acute injury from a commercial energy drink. The components of the energy drink that were responsible for the severe acute liver injury were not clear. The product name of the energy drink was not provided, but the ingredients listed in the report included vitamins, minerals and an “energy blend” which was likely to include caffeine, but other herbal components were not given.
Case 2. Acute hepatitis attributed to use of energy drinks.(2)
A 50 year old previously healthy man developed nausea, anorexia, epigastric pain and fatigue followed by dark urine and jaundice and was found to have markedly abnormal liver tests. He reported that he had been drinking 4 to 5 cans of an energy drink for the previous 3 weeks. He denied a history of liver disease, drug allergies, alcohol abuse, injection drug use or risk factors for viral hepatitis. He was not taking other medications or over-the-counter products. Initially, total serum bilirubin was 10.3 mg/dL (direct 7.7), ALT 1203 U/L, AST 1802 U/L, alkaline phosphatase 206 U/L, and INR 1.0. Tests for acute hepatitis A, B and E were negative, but he was positive for both anti-HCV and HCV RNA (5.6 million IU/mL). Antinuclear and smooth muscle antibodies were negative. Ultrasound of the abdomen showed no gallstones or evidence of biliary obstruction. A liver biopsy showed acute hepatitis with bridging necrosis and cholestasis but no fibrosis. Despite the HCV serology, he was diagnosed as having liver injury due to consumption of energy drinks, the suspected ingredient being niacin. After worsening for a few days, he began to improve spontaneously (Table) and in follow up weeks later he was asymptomatic and serum aminotransferase levels were normal. Follow up HCV RNA results were not reported.
Key Points
| Medication: | Energy drinks (4-5 cans daily for 3 weeks) |
|---|---|
| Pattern: | Hepatocellular (R=~18.8) |
| Severity: | 3+ (jaundice and Hospitalization) |
| Latency: | 3 weeks |
| Recovery: | Unclear in timing |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | AST (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|---|
| 3 weeks | 1 day | 1203 | 1802 | 206 | 10.3 | Hospitalized |
| 2 days | 1112 | 1710 | 11.5 | Anti-HCV and HCV RNA positive | ||
| 3 days | 1368 | 2435 | 13.4 | Liver biopsy | ||
| 4 days | 2073 | 4051 | 19.3 | |||
| 5 days | 1629 | 2674 | 15.7 | |||
| 6 days | 1224 | 1695 | 12.0 | Discharged, asymptomatic | ||
| 5 weeks | 14 days | 90 | 35 | 2.7 | ||
| 18 days | 23 | 36 | 2.2 | |||
| Normal Values | <38 | <42 | <130 | <1.2 | ||
Comment
This case report described a 50 year old construction worker who presented with acute hepatitis three weeks after starting regular consumption of energy drinks (4 to 5 cans of an undeclared product daily). Importantly, he also tested positive for anti-HCV and HCV RNA despite having no risk factors for hepatitis C and no previous knowledge of being infected. The authors attributed the acute hepatitis to consumption of the energy drinks and not to hepatitis C, arguing that he more likely had an asymptomatic chronic HCV infection. However, the clinical presentation, liver biopsy findings and subsequent course are entirely compatible with acute hepatitis C. The challenge in this diagnosis is that serologic tests (including IgM anti-HCV) are unreliable in separating acute from chronic infection, and definitive diagnosis of acute vs chronic HCV infection depends upon documentation of seroconversion to anti-HCV positivity. The diagnosis can also be fairly reliably made if the infection resolves as the hepatitis resolves with disappearance of HCV RNA (and rising titers of anti-HCV) or if there is a recent clear history of exposure. Such documentation, however, is not always available and a high proportion of patients with acute hepatitis C develop de novo chronic infection. In large case series, acute hepatitis C accounts for 1% to 5% of cases initially suspected to be drug induced liver injury. Of the six reported cases of acute liver injury from energy drink consumption published between 2000 and 2020, none were very convincing and most had other possible explanations (ischemic hepatitis, liver injury from other drugs being taken, acetaminophen or other toxic overdose).
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Energy Drinks – Monster®, NOS®, Red Bull®, Rockstar®
DRUG CLASS
CNS Stimulants, Xanthine Derivatives
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Caffeine | 58-08-2 | C8-H10-N4-O2 |
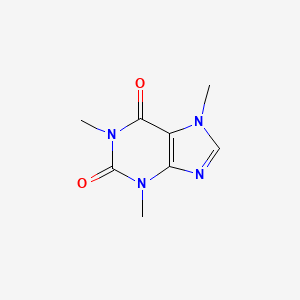
|
CITED REFERENCES
- 1.
- Vivekanandarajah A, Ni S, Waked A. Acute hepatitis in a woman following excessive ingestion of an energy drink: a case report. J Med Case Rep. 2011 Jun 22;5:227. [PMC free article: PMC3141691] [PubMed: 21696583]
- 2.
- Harb JN, Taylor ZA, Khullar V, Sattari M. Rare cause of acute hepatitis: a common energy drink. BMJ Case Rep. 2016;2016:bcr2016216612. [PMC free article: PMC5129143] [PubMed: 27803015]
ANNOTATED BIBLIOGRAPHY
References updated: 18 June 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999; caffeine and energy drinks are not discussed).
- Caffeine Informer. https://www
.caffeineinformer .com/the-caffeine-database. (Website on caffeine that provides information on the sources of caffeine, its clinical effects and side effects and a database on the caffeine content of coffees, teas, candy, foods, soft drinks and energy drinks). - O’Brien CP. Caffeine. In, Drug use disorders and addiction . In, Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 440.(Textbook of pharmacology and therapeutics).
- Winek CL, Wahba W, Williams K, Blenko J, Janssen J. Caffeine fatality: a case report. Forensic Sci Int. 1985;29(3-4):207–11. [PubMed: 4076952](21 year old woman took an overdose of 70 tablets that she thought were amobarbital but actually were mostly caffeine, and had a fatal cardiorespiratory arrest, autopsy demonstrating caffeine blood levels of 240 mg/L).
- Zimmerman PM, Pulliam J, Schwengels J, MacDonald SE. Caffeine intoxication: a near fatality. Ann Emerg Med. 1985;14:1227–9. [PubMed: 4061999](37 year old woman took an overdose of 27 gm of caffeine and rapidly developed hypotension, tachycardia, confusion, acidosis and coma [pH 7.2] followed by repeated episodes of ventricular fibrillation treated successfully with dialysis and medical support [initial caffeine level 199 mg/L], allowing for discharge 27 days later).
- Cannon ME, Cooke CT, McCarthy JS. Caffeine-induced cardiac arrhythmia: an unrecognised danger of healthfood products. Med J Aust. 2001;174:520–1. [PubMed: 11419773](25 year old woman with known mitral value prolapse had a fatal, witnessed cardiac arrest [ventricular fibrillation] having just consumed a large bottle of an energy drink [Race 2005: 300-570 mg of caffeine]; no mention of hepatic abnormalities).
- Holmgren P, Nordén-Pettersson L, Ahlner J. Caffeine fatalities--four case reports. Forensic Sci Int. 2004;139:71–3. [PubMed: 14687776](4 patients with fatal caffeine overdoses with lethal levels of caffeine in postmortem blood tests; no description of hepatic effects, although three had known risk factors for liver disease [alcoholism, drug abuse, hepatitis C]).
- Kerrigan S, Lindsey T. Fatal caffeine overdose: two case reports. Forensic Sci Int. 2005;153:67–9. [PubMed: 15935584](Two patients with fatal caffeine overdose, 39 year old woman with known drug use found dead and 29 year old male with obesity, diabetes and known overdose with caffeine pills and vomiting and seizures, had postmortem blood caffeine levels of 192 and 567 mg/L; no mention of liver abnormalities).
- Iyadurai SJ, Chung SS. New-onset seizures in adults: possible association with consumption of popular energy drinks. Epilepsy Behav. 2007;10:504–8. [PubMed: 17349826](Four adults had new onset seizures accompanied by transient tachycardia and hypertension shortly after consuming energy drinks [Rockstar, Monster], usually on an empty stomach or with diet pills and not recurring during follow up when the energy drinks were stopped; all four had no “laboratory abnormalities” except for high glucose in one).
- Haller C, Kearney T, Bent S, Ko R, Benowitz N, Olson K. Dietary supplement adverse events: report of a one-year poison center surveillance project. J Med Toxicol. 2008;4:84–92. [PMC free article: PMC3550135] [PubMed: 18570167](Among 275 poison control center calls about dietary supplements occurring over a 12 month period, 112 [41%] were symptomatic, 8 resulted in hospitalization and one was fatal, the majority of symptomatic cases [47%] were related to a caffeine-containing multiingredient products; no mention of hepatotoxicity).
- Berger AJ, Alford K. Cardiac arrest in a young man following excess consumption of caffeinated "energy drinks". Med J Aust. 2009;190:41–3. [PubMed: 19120009](28 year old man had cardiac arrest [ventricular fibrillation] shortly after consuming 7-8 cans of an energy drink [~640 mg of caffeine] and underwent extensive evaluation including coronary angiography which was normal; no mention of liver test abnormalities).
- Higgins JP, Tuttle TD, Higgins CL. Energy beverages: content and safety. Mayo Clin Proc. 2010;85:1033–41. [PMC free article: PMC2966367] [PubMed: 21037046](Review of the increasing popularity of energy drinks which often have high levels of caffeine [50-505 mg/serving] but are not regulated in regard to caffeine concentration or claims for performance enhancement; other ingredients may include glucose, taurine, branched chain amino acids, various vitamins, ginseng, guarana, ginkgo, milk thistle, L-carnitine and citric acid).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 12 [9%] attributed to herbals, but none to energy drinks).
- Vivekanandarajah A, Ni S, Waked A. Acute hepatitis in a woman following excessive ingestion of an energy drink: a case report. J Med Case Rep. 2011 Jun 22;5:227. [PMC free article: PMC3141691] [PubMed: 21696583](22 year old woman developed abdominal pain, nausea and vomiting having consumed 10 cans of energy drinks daily for 2 weeks [bilirubin 1.7 rising to 3.5 mg/dL, ALT 216 rising to 7553 U/L, Alk P and GGT normal, INR 1.6], recovering within the next few weeks with no specific therapy: Case 1).
- Apestegui CA, Julliard O, Ciccarelli O, Duc DK, Lerut J. Energy drinks: another red flag for the liver allograft. Liver Transpl. 2011;17:1117–8. [PubMed: 21674755](16 year old man with liver transplant developed sudden rise in liver tests on two occasions one year apart after consuming 3-5 cans of Red Bull daily [ALT 6 and 26 times ULN, bilirubin peak of 2.3 and 10.7 mg/dL], resolving in 4 to 8 months).
- Trabulo D, Marques S, Pedroso E. Caffeinated energy drink intoxication. BMJ Case Rep. 2011;2011:bcr0920103322. [PMC free article: PMC3062360] [PubMed: 22714613](28 year old man had sudden onset of seizures followed by lactic acidosis, coma and respiratory arrest shortly after drinking several cans of an energy drink [Red Bull] together with coffee, recovering within a week with medical support; no mention of liver test abnormalities although he had a history of injection drug use and hepatitis C).
- Babu KM, Zuckerman MD, Cherkes JK, Hack JB. First-onset seizure after use of an energy drink. Pediatr Emerg Care. 2011;27:539–40. [PubMed: 21642791](15 year old adolescent boy developed seizures after drinking 2 bottles of “5-hour ENERGY” with emesis and tachycardia [caffeine level 99 mg/L]; he had no further seizures in follow up without anticonvulsant therapy).
- Calabrò RS, Italiano D, Gervasi G, Bramanti P. Single tonic-clonic seizure after energy drink abuse. Epilepsy Behav. 2012;23:384–5. [PubMed: 22370117](20 year old man had a single seizure having consumed 4-6 cans of Red Bull daily for 5 months and had no further seizures in follow up after stopping energy drink consumption).
- Wolk BJ, Ganetsky M, Babu KM. Toxicity of energy drinks. Curr Opin Pediatr. 2012;24:243–51. [PubMed: 22426157](Review of the literature on adverse events linked to consumption of energy drinks including cardiovascular [arrhythmias, sudden death], renal [acute renal failure], hepatic [acute hepatitis], neurologic [seizures] and psychiatric effects [anxiety, poor sleep, developmental problems], the ingredients responsible for the adverse events not always being attributable to caffeine).
- Sepkowitz KA. Energy drinks and caffeine-related adverse effects. JAMA. 2013;309:243–4. [PubMed: 23330171](History of FDA actions in limiting and regulating caffeine containing products, including warning letters concerning energy drinks, mentions that many products do not list the concentration of caffeine and that there may be great individual variation in sensitivity or pharmacodynamics of caffeine which can be affected by underlying liver disease, alcohol use and medications that are metabolized via CYP 1A2).
- Noff T, Insel J. Energy drinks and the unwanted buzz: a case report. Md Med. 2013;13:28–9. [PubMed: 23556369](46 year old woman with coronary artery disease developed severe chest pain having consumed 3-5 energy drinks daily for 6 months and was found to have abnormal serum aminotransferase levels [bilirubin normal, ALT 325 U/L, AST 437 U/L] which the authors attributed to niacin [30 mg per serving] in the energy drinks; no further details provided).
- Consumption of Caffeine in Food and Dietary Supplements; Food and Nutrition Board; Board on Health Sciences Policy; Institute of Medicine. Caffeine in Food and Dietary Supplements: Examining Safety: Workshop Summary. Washington (DC): National Academies Press (US); 2014 Apr 23. [PubMed: 24872990](Summary of a workshop on the consumption of caffeine in the United States and evidence for and against cardiovascular and central nervous system safety; no discussion of liver toxicity).
- Huang B, Kunkel D, Kabany ME. Acute liver failure following one year of daily consumption of a sugar-free energy drink. ACG Case Rep J. 2014;1(4):214–6. [PMC free article: PMC4435335] [PubMed: 26157880](36 year old man developed jaundice having consumed 3 energy drinks [Rockstar] daily for a year and having a 15 year history of binge alcohol drinking [bilirubin 16.1 mg/dL, ALT 2995 U/L, Alk P 231 U/L, INR 1.0], with subsequent worsening [bilirubin rising to 23.1 mg/dL, INR 3.7, hepatic encephalopathy], ultimately requiring and undergoing a successful liver transplantation).
- Eichner ER. Fatal caffeine overdose and other risks from dietary supplements. Curr Sports Med Rep. 2014;13:353–4. [PubMed: 25391087](Editorial on the dangers including fatalities of unregulated dietary supplements that may contain excessive amounts of caffeine or have toxicities due to contaminants such as amphetamines [Frenzy, Craze] or aegeline [OxyELITE Pro]).
- Gurley BJ, Steelman SC, Thomas SL. Multi-ingredient, caffeine-containing dietary supplements: history, safety, and efficacy. Clin Ther. 2015;37:275–301. [PubMed: 25262198](Extensive review of the history, clinical efficacy and safety of multiingredient, caffeine containing dietary supplements which have been widely used as stimulants and weight loss agents and may have greater cardiovascular and neurologic adverse effects because of increase in caffeine toxicity caused by interaction with other components in the supplements such as taurine, p-synephrine, yohimbine, green tea, theanine, dimethylamylamine [DMAA], diethylphenylethylamine [DEPEA] and, previously, ephedra).
- Harb JN, Taylor ZA, Khullar V, Sattari M. Rare cause of acute hepatitis: a common energy drink. BMJ Case Rep. 2016;2016:bcr2016216612. [PMC free article: PMC5129143] [PubMed: 27803015](50 year old man developed an acute hepatitis 3 weeks after consuming 4-5 energy drinks daily [bilirubin 10.3 rising to 19.3 mg/dL, ALT 1203 U/L, Alk P 206 U/L, anti-HCV and HCV RNA positive], with rapid resolution on stopping the energy drinks but no information on HCV RNA levels in follow up: Case 2).
- Temple JL, Bernard C, Lipshultz SE, Czachor JD, Westphal JA, Mestre MA. The safety of ingested caffeine: a comprehensive review. Front Psychiatry. 2017;8:80. [PMC free article: PMC5445139] [PubMed: 28603504](Review of the safety of caffeine for healthy as well as vulnerable populations concludes that daily intake of up to 400 mg of caffeine is probably safe for healthy adults, but lower levels should be recommended for children and pregnant women).
- Brown AC. Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem Toxicol 2017; 107(Pt A): 472-501. [PubMed: 27402097](Description of an online compendium of cases of liver toxicity attributed to HDS products, lists published cases for multiple agents, but energy drinks are not discussed).
- Wong LL, Lacar L, Roytman M, Orloff SL. Urgent liver transplantation for dietary supplements: an under-recognized problem. Transplant Proc. 2017;49:322–5. [PubMed: 28219592](Among 2048 adult liver transplants recipients enrolled in the Scientific Registry of Transplant Recipients [SRTR] between 2003 and 2015, 625 were done for acute hepatic necrosis due to drug induced liver injury, half being due to acetaminophen and the 4th most frequent cause [n=21] being HDS products, of which 5 were attributed to OxyELITE Pro, but none to caffeine or energy drinks).
- Higgins JP, Babu K, Deuster PA, Shearer J. Energy drinks: a contemporary issues paper. Curr Sports Med Rep. 2018;17:65–72. [PubMed: 29420350](Review of the definition, efficacy and safety of energy drinks which have become increasingly popular, particular among high school and college students, athletes and body builders, and are defined as high caffeine containing beverages that usually contain miscellaneous other components such as vitamins, minerals, amino acids and herbal products such as ginseng, yohimbe and ginkgo; adverse effects are largely attributable to caffeine and include neurologic and cardiovascular effects, with only rare, isolated reports of liver or kidney injury).
- Harty PS, Zabriskie HA, Erickson JL, Molling PE, Kerksick CM, Jagim AR. Multi-ingredient pre-workout supplements, safety implications, and performance outcomes: a brief review. J Int Soc Sports Nutr. 2018;15:41. [PMC free article: PMC6083567] [PubMed: 30089501](Review of the efficacy and safety of multiingredient pre-workout supplements which typically contain caffeine, amino acids, nitrates and creatine and are “relatively safe with minimal reported adverse events”, mentions that their use has not been linked to serum enzyme elevations; no discussion of hepatotoxicity or concurrent use of anabolic steroids).
- Al Yacoub R, Luczkiewicz D, Kerr C. Acute kidney injury and hepatitis associated with energy drink consumption: a case report. J Med Case Rep. 2020;14(1):23. [PMC free article: PMC6988357] [PubMed: 31992329](62 year old woman with advanced small cell lung cancer in hospice developed nausea, vomiting and confusion and was found to have abnormal liver tests [bilirubin 0.3 mg/dL, ALT 2866 U/L, AST 4333 U/L, Alk P 111 U/L], having had little oral intake for 2 weeks except for 5-6 cans of an energy drink daily).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review International society of sports nutrition position stand: energy drinks and energy shots.[J Int Soc Sports Nutr. 2023]Review International society of sports nutrition position stand: energy drinks and energy shots.Jagim AR, Harty PS, Tinsley GM, Kerksick CM, Gonzalez AM, Kreider RB, Arent SM, Jager R, Smith-Ryan AE, Stout JR, et al. J Int Soc Sports Nutr. 2023 Dec; 20(1):2171314.
- Prevalence and Amounts of Common Ingredients Found in Energy Drinks and Shots.[Nutrients. 2022]Prevalence and Amounts of Common Ingredients Found in Energy Drinks and Shots.Jagim AR, Harty PS, Barakat AR, Erickson JL, Carvalho V, Khurelbaatar C, Camic CL, Kerksick CM. Nutrients. 2022 Jan 13; 14(2). Epub 2022 Jan 13.
- Review [Risks of energy drinks in youths].[Arch Pediatr. 2010]Review [Risks of energy drinks in youths].Bigard AX. Arch Pediatr. 2010 Nov; 17(11):1625-31.
- Changing beverage consumption patterns have resulted in fewer liquid calories in the diets of US children: National Health and Nutrition Examination Survey 2001-2010.[J Acad Nutr Diet. 2015]Changing beverage consumption patterns have resulted in fewer liquid calories in the diets of US children: National Health and Nutrition Examination Survey 2001-2010.Mesirow MS, Welsh JA. J Acad Nutr Diet. 2015 Apr; 115(4):559-66.e4. Epub 2014 Nov 7.
- Update on energy drinks and youth.[J Psychosoc Nurs Ment Health S...]Update on energy drinks and youth.Fogger S, McGuinness TM. J Psychosoc Nurs Ment Health Serv. 2011 Dec; 49(12):17-9. Epub 2011 Nov 16.
- Energy Drinks - LiverToxEnergy Drinks - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...