NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Theophylline is an orally administered xanthine derivative that induces relaxation of smooth muscle in the bronchial tree causing bronchodilation. Theophylline is widely used in therapy of asthma and is not believed to cause liver injury.
Background
Theophylline (thee of' i lin) is a methylxanthine related structurally to caffeine and has been widely used as a bronchodilator since the 1930s. Oral theophylline was commonly used in treatment of acute attacks of asthma and in chronic management of bronchospasm in patients with asthma and chronic obstructive pulmonary disease, but has been declining in use with the development of more effective and better tolerated inhalation therapies. Theophylline was formally approved for use in the United States in 1982 and is available in multiple generic forms for oral and intravenous use. Typical capsule or tablet sizes are 100, 200 and 300 mg. Typical dose regimens are 100 to 300 mg three to four times daily. Syrups are available for use in children. Extended release formulations are also available in sizes of 100 to 600 mg which are typically given twice daily. Dosage is highly individualized. Intravenous formations are available for management of acute asthmatic attacks. Theophylline is marketed under multiple brand names including Asmalix, Elixophyllin, Quibron-T, Respbid, Theobid, Duracaps, and Uniphyl. Common side effects of theophylline include dizziness, headache, insomnia, restlessness, tachycardia, palpitations, flushing, nausea, abdominal discomfort and diaphoresis, largely due to the effects of theophylline on the CNS, heart and muscle tissue. In high doses, theophylline can cause irritability, tremors and seizures as well as tachycardia, cardiac arrhythmias and sudden death. With the development of safer, better tolerated and more effective inhaled bronchodilators, oral theophylline has become less frequently used.
Hepatotoxicity
Prospective studies have shown that ALT elevations occur in less than 1% of patients receiving long term oral therapy with theophylline, making it unlikely that such elevations are more common than might occur by chance. No instances of clinically apparent liver injury have been convincingly linked to theophylline use. Liver injury may occur as a part of generalized toxicity from high doses of theophylline probably as a result of hepatic complications of hypotension or anoxia from seizures or cardiac arrhythmias caused by the drug. Theophylline actually has been shown to attenuate several forms of experimental acute liver injury, and chronic caffeine (another xanthine) intake has been associated with decreased rates of chronic liver disease, cirrhosis and liver cancer.
Likelihood score: E (unlikely cause of clinically apparent liver injury in conventional oral doses).
Mechanism of Liver Injury
Theophylline is metabolized in the liver and levels are sensitive to stimulation or inhibition of the P450 enzyme system and levels are increased by many drugs such as cimetidine, corticosteroids, macrolide and quinolone antibiotics, and interferon. Theophylline levels may be decreased by barbiturates, phenytoin and rifampicin. Similarly, theophylline may affect the metabolism of other drugs and care must be used in adding theophylline in patients on multiple medications.
Outcome and Management
Typically, liver enzymes abnormalities that occur during therapy resolve rapidly even when theophylline is continued. Clinically apparent liver injury with jaundice does not occur or is too rare to make any statements about severity or prognosis.
Drug Class: Antiasthmatic Agents, Xanthine Derivatives
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Theophylline – Generic, Slo-Phyllin®, Thio-Dur®, Uniphyl®
DRUG CLASS
Antiasthmatic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
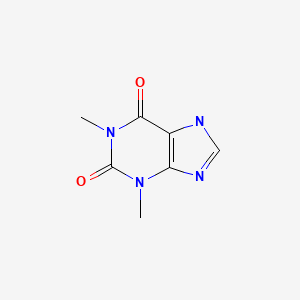
| Theophylline | 58-55-9 | C7-H8-N4-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 18 July 2020
- Zimmerman HJ. Respiratory supportive drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, p. 717.(Expert review of hepatotoxicity published in 1999; mentions that theophylline has been incriminated in hepatic injury rarely; no mention of montelukast, zafirlukast or zileuton).
- Barnes PJ. Pulmonary pharmacology. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 727-49.(Textbook of pharmacology and therapeutics).
- Strubelt O, Wegener F, Siegers CP. Arzneimittelforschung. 1970;20:473–6. [Hepatotoxicity of caffeine and theophylline] German. [PubMed: 5467807](Administration of coffee or theophylline to rats sc and ip resulted in increases in serum free fatty acids levels and minimal increases in ALT and AST).
- Staib AH, Schuppan D, Lissner R, Zilly W, von Bomhard G, Richter E. Pharmacokinetics and metabolism of theophylline in patients with liver diseases. Int J Clin Pharmacol Ther Toxicol. 1980;18:500–2. [PubMed: 7203726](Study of metabolism of single iv dose of theophylline in patients with various forms of liver disease showed only patients with decompensated cirrhosis had significantly lower clearance rates that might lead to higher plasma levels).
- Woodcock AA, Johnson MA, Geddes DM. Theophylline prescribing, serum concentrations, and toxicity. Lancet. 1983;2:610–3. [PubMed: 6136756](Monitoring theophylline levels found high levels in 13 outpatient and 4 cases of serious adverse events and 3 deaths [seizures and cardiac arrest], but no frank clinical hepatotoxicity).
- Piperno D, Pacheco Y, Bastion Y, Favre-Monet PY, Gormand F, Copere B, Kofman J, et al. Therapie. 1988;43:481–3. [Theophylline-induced hepatitis. Apropos of 2 cases] French. [PubMed: 3067422](65 year old woman and 69 year old man with asthma developed ALT elevations without jaundice 11 and 4 days after starting intravenous theophylline (peak ALT 520 and 1345 U/L), and positive rechallenge in one patient).
- Drent M, Kuks PF, van den Bosch JM, Brandt KH. Ned Tijdschr Geneeskd. 1993;137:1831–5. [Psychiatric symptoms due to theophylline overdose; the necessity of blood level monitoring] Dutch. [PubMed: 8377864](Chronic overdosing with theophylline led to severe psychiatric symptoms and depression; one patient developed hypotension and ischemic hepatitis [ALT and LDH ~5000 U/L without mention of jaundice]).
- Makino S, Adachi M, Ohta K, Kihara N, Nakajima S, Nishima S, Fukuda T, et al. A prospective survey on safety of sustained-release theophylline in treatment of asthma and COPD. Allergol Int. 2006;55:395–402. [PubMed: 17130682](Prospective study of 3921 patients at 66 centers receiving sustained release theophylline found no serious adverse events; only 1 patient with ALT elevation).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, one case was attributed to zafirlukast, but no other antiasthma medication mentioned).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J. Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to an antiasthma medication).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to theophylline or any other medications for asthma).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 4 cases [<1%] were attributed to medications for asthma, all due to montelukast and none to theophylline, zafirlukast or zileuton).
- Drugs for asthma. Med Lett Drugs Ther. 2017;59(1528):139–46. [PubMed: 28880849](Concise review of medications used for asthma including theophylline; mentions that theophylline has been replaced by safer and better tolerated agents and that side effects include nausea, vomiting, headache and insomnia and with high doses, tachycardia, cardiac arrhythmias, irritability, tremors, seizures and even sudden death; no mention of liver injury).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Methylxanthines in asthma.[Handb Exp Pharmacol. 2011]Review Methylxanthines in asthma.Tilley SL. Handb Exp Pharmacol. 2011; (200):439-56.
- Review Theophylline and selective PDE inhibitors as bronchodilators and smooth muscle relaxants.[Eur Respir J. 1995]Review Theophylline and selective PDE inhibitors as bronchodilators and smooth muscle relaxants.Rabe KF, Magnussen H, Dent G. Eur Respir J. 1995 Apr; 8(4):637-42.
- Effect of theophylline and enprofylline on bronchial hyperresponsiveness.[Thorax. 1989]Effect of theophylline and enprofylline on bronchial hyperresponsiveness.Koëter GH, Kraan J, Boorsma M, Jonkman JH, van der Mark TW. Thorax. 1989 Dec; 44(12):1022-6.
- Effects of adenosine and theophylline on canine tracheal smooth muscle tone.[Arch Int Pharmacodyn Ther. 1987]Effects of adenosine and theophylline on canine tracheal smooth muscle tone.Krzanowski JJ, Urdaneta-Bohorquez A, Polson JB, Sakamoto Y, Szentivanyi A. Arch Int Pharmacodyn Ther. 1987 Jun; 287(2):224-36.
- Review Xanthine Derivatives.[LiverTox: Clinical and Researc...]Review Xanthine Derivatives.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Theophylline - LiverToxTheophylline - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...