NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Thiabendazole is a broad spectrum anthelmintic agent used predominantly in treatment of intestinal pinworm and strongyloides infection, which recently has been replaced by better tolerated agents. Thiabendazole therapy has been shown to cause clinically apparent cholestatic liver injury which is rare, but can be severe.
Background
Thiabendazole (thye" a ben' da zole) is a benzimidazole anthelmintic agent similar in structure and mechanism of action to albendazole and mebendazole. The benzimidazoles act by selective binding to beta-tubulin of parasitic worms, causing their immobilization and death. Thiabendazole was approved in the United States in 1967, but has subsequently been withdrawn because of the availability of other, better tolerated anthelmintic agents, such as ivermectin, albendazole and mebendazole. Thiabendazole is, however, still available in other countries and is used in veterinary medicine in the United States. Thiabendazole is also used as a food preservative and fungicide and trace amounts can be detected in some fruit juices and animal products. Its major medical indication is strongyloidiasis infestation. Thiabendazole was formerly available in chewable tablets of 500 mg under the trade name of Mintezol and as an oral suspension. The typical dose in adults was 1500 mg orally daily for 1 to 3 days. The dose in children is based upon body weight. Thiabendazole therapy is associated with frequent minor but troublesome side effects including dizziness, nausea, diarrhea, gastrointestinal upset, headaches and hair loss. Severe adverse events can include liver disease, keratoconjunctivity sicca, photosensitivity, skin rash and hypersensitivity reactions.
Hepatotoxicity
Thiabendazole therapy is associated with serum aminotransferase elevations in up to 36% of patients, but it is usually given for a brief period only and its effects on serum enzyme levels after single dose administration has not been systematically evaluated. Importantly, thiabendazole therapy has also been associated with clinically apparent liver injury which can be prolonged and severe. The onset of injury is usually within 1 to 2 weeks of finishing a 1 to 5 day course of therapy. The pattern of serum enzyme elevations is typically cholestatic. Autoantibodies are usually negative and fever, arthralgias and rash are uncommon. Several reported cases have been associated with sicca complex marked by parotid enlargement and tenderness, dry eyes and dry mouth arising before the onset of jaundice (Case 1). The cholestatic injury can be associated with damage to small bile ducts and with prolonged jaundice and/or pruritus and alkaline phosphatase elevations. Several instances of prolonged cholestasis and chronic vanishing bile duct syndrome and end stage liver disease has been reported even after a single dose of thiabendazole.
Mechanism of Injury
Thiabendazole hepatotoxicity appears to be due to an immunological reaction to the drug, which is directed largely at bile, lacrimal, and salivary gland ducts.
Outcome and Management
While most reported cases of thiabendazole induced liver injury were self-limited, many were marked by severe and prolonged cholestasis. Several cases of prolonged jaundice with vanishing bile duct syndrome and evolution to cirrhosis leading to liver transplantation have been reported. Patients with acute liver injury attributed to thiabendazole should avoid repeat exposure. It is unknown whether there is cross sensitivity with other benzimidazoles (such as albendazole and mebendazole), but there probably is and switching to another class of anthelmintic agents is appropriate if therapy is still needed.
Drug Class: Anthelmintic Agents
CASE REPORT
Case 1. Cholestatic hepatitis followed by chronic cholestatic syndrome and cirrhosis after a short course of thiabendazole.(1)
A 46 year old woman with suspected trichinosis was treated with thiabendazole (25 mg/kg twice daily for 5 days) and prednisone (40 mg/day). Two weeks later, she noted dryness of the eyes and mouth with low grade fever. Shortly thereafter she developed dark urine and pruritus. She had no history of liver disease or drug allergies and drank little alcohol. On admission, she had prominence of the parotid glands and right upper quadrant tenderness without enlargement of the liver or spleen. She was jaundiced but had no other signs of chronic liver disease. Laboratory values showed a total bilirubin of 16.4 mg/dL with a direct of 11.5 mg/dL (Table). Serum aminotransferase levels were 3 to 20 fold elevated and 5’ nucleotidase was 5 times elevated. Tests for hepatitis A and B and routine autoantibodies were normal. Ultrasound and CT scans of the abdomen showed no abnormalities and endoscopic retrograde cholangiopancreatography revealed no evidence of biliary obstruction. A liver biopsy showed marked intrahepatic cholestasis and a relative paucity of bile ducts. A salivary gland biopsy showed sialadenitis with prominent destruction of acini and ductules. She remained deeply jaundiced, but reevaluation showed no evidence for other liver disease. Over the next 8 months her symptoms of dry mouth and eyes improved, but she continued to have pruritus and mild jaundice. Two and a half years after exposure to thiabendazole she developed ascites and variceal bleeding. A repeat liver biopsy showed a micronodular cirrhosis with little inflammation and no steatosis. She remained negative for antinuclear (ANA), antimitochondrial (AMA), and antimicrosomal antibodies. Shortly thereafter, she underwent liver transplantation; the explant again showed micronodular cirrhosis.
Key Points
| Medication: | Thiabendazole (25 mg/kg twice daily for 5 days) |
|---|---|
| Pattern: | Cholestatic pattern (R=~0.8) |
| Severity: | 5+ (ultimately leading to cirrhosis and liver transplantation) |
| Latency: | 2 weeks |
| Recovery: | Incomplete |
| Other medications: | Prednisone 40 mg daily for short period |
Laboratory Values
Comment
Thiabendazole typically causes an acute cholestatic hepatitis, but in half of cases the cholestatic hepatitis has been preceded by onset of a sicca syndrome with dry eyes and dry mouth and parotid enlargement, with salivary gland biopsies showing inflammation and damage to acini and ductules. The cholestatic hepatitis may also be characterized by damage to bile ducts and result in a prolonged jaundice or cholestasis, with persistence of liver test abnormalities for months or years. In its most severe form, this prolonged cholestasis is also marked by vanishing bile duct syndrome and chronic cholestasis that can result in cirrhosis, portal hypertension and end stage liver disease. The condition resembles primary biliary cirrhosis, but typically presents in an acute fashion with jaundice and symptoms, whereas primary biliary cirrhosis usually presents insidiously with liver test abnormalities and pruritus long before onset of jaundice. Interestingly, primary biliary cirrhosis can also be associated with keratoconjunctivitis sicca, and the histology of the involved salivary and lacrimal glands resembles that of primary biliary cirrhosis with intense inflammation and damage to small bile ducts, with rupture and granulomatous inflammation in the acute phase. This striking syndrome, several cases of which have been reported after short courses of thiabendazole, has not been reported with the other anthelmintic benzimidazoles, mebendazole and albendazole.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Thiabendazole – Generic, Mintezol®
DRUG CLASS
Anthelmintic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Thiabendazole | 148-79-8 | C10-H7-N3-S |
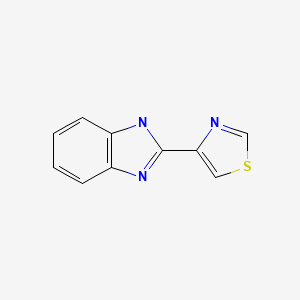
|
CITED REFERENCE
- 1.
- Roy MA, Nugent FW, Aretz HT. Micronodular cirrhosis after thiabendazole. Dig Dis Sci. 1989;34:938–41. [PubMed: 2721325]
ANNOTATED BIBLIOGRAPHY
References updated: 18 September 2021
- Zimmerman HJ. Anthelminthics. Hepatic injury from antimicrobial agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 626-8.(Expert review of hepatotoxicity of anthelmintics written in 1999; mentions that thiabendazole has been associated with at least 17 instances of cholestatic jaundice).
- Keiser J, McCarthy J, Hotez P. Chemotherapy of helminth infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1001-9.(Textbook of pharmacology and therapeutics; albendazole and mebendazole are discussed but not thiabendazole).
- Hennekeuser HH, Pabst K, Poeplau W, Gerok W. Thiabendazole for the treatment of trichinosis in humans. Tex Rep Biol Med. 1969;27 Suppl 2:582–96. [PubMed: 5369623](Among 23 patients with trichinosis [during an epidemic in Germany], treated with thiabendazole [50 mg/kg/day] for 10 days, one patient [4%] developed liver injury with ALT and AST elevations and biopsy showing hepatocellular necrosis and cholestasis; no further information given).
- Bayles MAH. Chromomycosis: treatment with thiabendazole. Arch Dermatol. 1971;104:476–85. [PubMed: 5120175](Among 14 patients with chromomycosis treated with thiabendazole [25 mg/kg/day] long term, 5 developed toxic-allergic hepatitis arising after 1-18 months of therapy, 4 with jaundice, 1 died; rapid recurrence on restarting; few details given).
- Jalota R, Freston JW. Severe intrahepatic cholestasis due to thiabendazole. Am J Trop Med Hyg. 1974;23:676–8. [PubMed: 4847042](32 year old woman developed fever and rash a day after finishing 8 day course of thiabendazole for pinworms, with subsequent jaundice [bilirubin rising to 23 mg/dL, AST 123 U/L, Alk P 209 U/L]; liver biopsy showed intrahepatic cholestasis, but she died of hemorrhage, a complication of transhepatic cholangiogram).
- Feregrino Goyos M, Lifshitz Guinzberg A, Hernández Berumen A, Ramírez Degollado J, Cervantes LF. Prensa Med Mex. 1976;41:167–71. [Intrahepatic cholestasis by thiabendazole treated with phenobarbital] Spanish. [PubMed: 995842]
- Fink AI, MacKay CJ, Cutler SS. Sicca complex and cholestatic jaundice in two members of a family caused by thiabendazole. Trans Am Ophthalmol Soc. 1978;76:108–15. [PMC free article: PMC1311616] [PubMed: 754367](Mother and daughter developed sicca syndrome followed by jaundice after 5 members of family were given 2 doses of thiabendazole for pinworms; mother, aged 43, developed symptoms at 2 weeks with parotid enlargement, dry mouth and eyes, and jaundice 1 week later [peak bilirubin 15.7 mg/dL, Alk P 944 U/L, AST 200 U/L], resolving in 1 month; child, aged 17, had onset of symptoms in 1 week and jaundice in 2, with prolonged jaundice, pruritus and dry eyes, ultimately resolving).
- Fink AI, MacKay CJ, Cutler SS. Sicca complex and cholangiostatic jaundice in two members of a family probably caused by thiabendazole. Ophthalmology. 1979;86:1892–6. [PubMed: 553260](Same cases as described by the authors in Trans Am Ophthalmol Soc 1978).
- Rex D, Lumeng L, Eble J, Rex L. Intrahepatic cholestasis and sicca complex after thiabendazole. Report of a case and review of the literature. Gastroenterology. 1983;85:718–21. [PubMed: 6873616](55 year old man developed jaundice 8 days after starting a 3 day course of thiabendazole for strongyloidiasis [bilirubin 30.6 rising to 40.5 mg/dL, AST 562 U/L, Alk P 820 U/L], and dry eyes, but normal salivary gland biopsy, liver biopsy showing intrahepatic cholestasis without duct loss, prolonged, severe course [confusion and edema], but ultimate recovery).
- Manivel JC, Bloomer JR, Snover DC. Progressive bile duct injury after thiabendazole administration. Gastroenterology. 1987;93:245–9. [PubMed: 3297909](27 year old man developed jaundice 2 weeks after finishing 1 week course of thiabendazole for suspected strongyloidiasis [peak bilirubin 24.5 mg/dL, ALT 328 U/L, Alk P 6280 U/L], liver biopsies showing duct injury and loss, followed by intractable pruritus and liver failure and liver transplant 3 years after onset).
- Davidson RN, Weir WR, Kaye GL, McIntyre N. Intrahepatic cholestasis after thiabendazole. Trans R Soc Trop Med Hyg. 1988;82:620. [PubMed: 3256116](17 year old boy developed dry mouth 1 day after finishing 3 day course of thiabendazole for strongyloides infection, becoming jaundiced 2 weeks later [bilirubin rising to 23.8 mg/dL, ALT 102 U/L, Alk P 350 U/L], biopsy showed severe intrahepatic cholestasis, jaundice resolved in 6 and serum enzyme elevations in 12 months).
- Roy MA, Nugent FW, Aretz HT. Micronodular cirrhosis after thiabendazole. Dig Dis Sci. 1989;34:938–41. [PubMed: 2721325](46 year old woman developed dry eyes and mouth 2 weeks after a 5 day course of thiabendazole for trichinosis, followed by jaundice and pruritus 1 week later [bilirubin 16.4 mg/dL, ALT 487 U/L], liver biopsy showed intrahepatic cholestasis and paucity of bile ducts, with prolonged jaundice and pruritus and variceal hemorrhage 2.5 years later: Case 1).
- Bion E, Pariente EA, Maitre F. Severe cholestasis and sicca syndrome after thiabendazole. J Hepatol. 1995;23:762–3. [PubMed: 8750181](56 year old man developed severe dry mouth and eyes 2 weeks after a 3 day course of thiabendazole, followed 3 days later by jaundice [bilirubin rising to 27.2 mg/dL, ALT 5.5 times ULN, Alk P 8 times ULN, GGT 29 times ULN], liver biopsy showed intrahepatic cholestasis and salivary gland biopsy sialadenitis, slow recovery and persistent GGT elevation 6 years later).
- Skandrani K, Richardet JP, Duvoux C, Cherqui D, Zafrani ES, Dhumeaux D. Gastroenterol Clin Biol. 1997;21:623–5. [Hepatic transplantation for severe ductopenia related to ingestion of thiabendazole] French. [PubMed: 9587501](26 year old man developed dry eyes and dry mouth 4 days after finishing a second course of thiabendazole [25 mg/kg for 3 days] for strongyloidiasis, with subsequent jaundice [bilirubin 18.3 mg/dL, ALT 2.5 times ULN, Alk P 1.5 times ULN], liver biopsy showed intrahepatic cholestasis and loss of bile ducts; subsequent persistent severe cholestasis evolving into cirrhosis; liver transplant 1.5 years after onset).
- Eland IA, Kerkhof SC, Overbosch D, Wismans PJ, Stricker BH. Ned Tijdschr Geneeskd. 1998;142:1331–4. [Cholestatic hepatitis ascribed to the use of thiabendazole] Dutch. [PubMed: 9752041](Two women with cholestatic hepatitis arising 2-3 weeks after 2 day course of thiabendazole for strongyloidiasis, with slow and incomplete recovery).
- Drugs for parasitic infections. Treat Guidel Med Lett. 2013;11 Suppl:e1–31.(Brief description of drugs for parasitic infections in adults and children as well as a table of their major side effects; thiabendazole is mentioned as a drug of choice but is not discussed).
- Bisoffi Z, Buonfrate D, Angheben A, Boscolo M, Anselmi M, Marocco S, Monteiro G, et al. Randomized clinical trial on ivermectin versus thiabendazole for the treatment of strongyloidiasis. PLoS Negl Trop Dis. 2011;5:e1254. [PMC free article: PMC3144183] [PubMed: 21814588](Controlled trial of thiabendazole [2 daily doses for 2 days] vs ivermectin [single dose] in 198 patients with Strongyloidiasis found similar efficacy, but fewer side effects with ivermectin; no mention of hepatotoxicity or ALT elevations).
- Groh M, Blanche P, Calmus Y, Guillevin L. Thiabendazole-induced acute liver failure requiring transplantation and subsequent diagnosis of polyarteritis nodosa. Clin Exp Rheumatol. 2012;30(1) Suppl 70:S107–9. [PubMed: 22640653](33 year old man with chronic hypereosinophilia developed severe liver injury after experimental therapy with thiabendazole which resulted in vanishing bile duct syndrome, hepatic failure and liver transplantation; few details given).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, only one of which was attributed to an anthelmintic, mebendazole; none were attributed to thiabendazole).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 409 [46%] were attributed to antimicrobial agents, but none to anthelmintics or to thiabendazole).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Comparison of various anthelmintic therapies for the treatment of Trypanoxyuris microon infection in owl monkeys (Aotus nancymae).[Comp Med. 2007]Comparison of various anthelmintic therapies for the treatment of Trypanoxyuris microon infection in owl monkeys (Aotus nancymae).Bentzel DE, Bacon DJ. Comp Med. 2007 Apr; 57(2):206-9.
- Development and Validation of a Fast Stability-Indicating Ion-Paired Reversed-Phase HPLC Method for the Assay of Thiabendazole and Estimation of Its Related Compounds.[J AOAC Int. 2017]Development and Validation of a Fast Stability-Indicating Ion-Paired Reversed-Phase HPLC Method for the Assay of Thiabendazole and Estimation of Its Related Compounds.Zhang P, Tian J, Rustum A. J AOAC Int. 2017 Jan 1; 100(1):74-81.
- [Cholestatic hepatitis ascribed to the use of thiabendazole].[Ned Tijdschr Geneeskd. 1998][Cholestatic hepatitis ascribed to the use of thiabendazole].Eland IA, Kerkhof SC, Overbosch D, Wismans PJ, Stricker BH. Ned Tijdschr Geneeskd. 1998 Jun 6; 142(23):1331-4.
- Review Anthelmintic Agents.[LiverTox: Clinical and Researc...]Review Anthelmintic Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection.[Cochrane Database Syst Rev. 2016]Review Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection.Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC Jr, Terashima A, Samalvides F, Pérez-Molina JA, Plana MN. Cochrane Database Syst Rev. 2016 Jan 18; 2016(1):CD007745. Epub 2016 Jan 18.
- Thiabendazole - LiverToxThiabendazole - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...