NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Gemcitabine is a cytosine analogue and intravenously administered antineoplastic agent used in the therapy of several forms of advanced, pancreatic, lung, breast, ovarian and bladder cancer. Gemcitabine is associated with a high rate of transient serum enzyme elevations during therapy but is a very rare cause of acute, clinically apparent liver injury.
Background
Gemcitabine (jem sye' ta been), 2,,2.-difluoro deoxycytidine, is a pyrimidine analogue that is widely used in solid tumor chemotherapy. Intracellularly, it is metabolized to diphosphate and triphosphate forms, both of which have antineoplastic activity inhibiting ribonucleotide reductase and competing with deoxycytidine triphosphate for incorporation into DNA. Gemcitabine is classified as an antimetabolite and is believed to act by inhibition of DNA synthesis in rapidly dividing cells. Gemcitabine was approved for use in cancer chemotherapy in the United States in 1996 and current indications include chemotherapy for advanced pancreatic, non-small cell lung, breast, ovarian and bladder cancers, either alone or in combination with other antineoplastic agents. Gemcitabine is administered intravenously and is available in vials of 10 and 50 mL in concentrations of 20 mg/mL generically and under the brand name Gemzar. The regimen of administration and dose vary by indication. A typical dosing regimen is 30 minute infusions of 1.0 to 1.2 g/m2 on days 1, 8 and 15 of each 28 day cycle of chemotherapy. Common side effects include bone marrow suppression, fatigue, diarrhea, nausea, gastrointestinal upset, rash, alopecia, and stomatitis.
Hepatotoxicity
Elevations in serum aminotransferase levels occur in 30% to 90% of patients receiving cyclic therapy with gemcitabine. The elevations are generally mild-to-moderate, asymptomatic and self-limited, frequently resolving without discontinuation or even interruption of therapy. ALT or AST elevations above 5 times the upper limit of the normal range occur in 1-4% of patients yet rarely lead to symptoms or clinically apparent liver injury. Serum bilirubin and alkaline phosphatase elevations are less common, but also typically transient and mild. Despite wide use, gemcitabine has only rarely been implicated in rare cases of acute liver injury with jaundice, and most published cases have been reported in patients with underlying chronic liver disease or extensive hepatic metastases. The clinical features of hepatotoxicity from gemcitabine have not been well described. Most cases were marked by a progressive cholestasis and hepatic failure developing after several cycles of therapy in patients with preexisting chronic liver disease (hepatitis C, alcoholic liver disease) or significant hepatic metastases or local invasion.
As with many antineoplastic agents and regimens, therapy with gemcitabine has also been associated rare cases of with reactivation of hepatitis B in persons with preexisting HBsAg in serum. At least one case of sinusoidal obstruction syndrome (veno-occlusive disease) has been reported with use of gemcitabine in a patient with underlying chronic hepatitis C who received no other antineoplastic agent.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The frequent mild-to-moderate serum aminotransferase elevations that occur during gemcitabine therapy are likely due to direct hepatic toxicity. The clinically apparent liver injury linked to gemcitabine (cholestasis, HBV reactivation, sinusoidal obstruction syndrome) has occurred mostly in persons with underlying, preexisting liver disease. Whether this injury is idiosyncratic or the result of mild direct hepatotoxicity superimposed on significant underlying liver dysfunction is unknown.
Outcome and Management
The severity of the liver injury linked to gemcitabine therapy is usually mild and self-limited, and dose modification or discontinuation is rarely necessary. However, the clinically apparent liver injury described with gemcitabine therapy tends to be severe and several fatal instances have been described. Virtually all of the clinically apparent liver injury related to gemcitabine has occurred in patients with underlying liver disease or extensive hepatic metastases or local invasion. In view of this, considerable care should be exercised in treating patients who have liver disease with gemcitabine. Furthermore, monitoring of routine liver tests before and during therapy is recommended with suspension of infusions if jaundice or clinically apparent liver injury arises. The possibility exists that the mild direct hepatotoxicity becomes clinically important in the presence of significant preexisting liver disease or dysfunction. The outcome of reexposure to gemcitabine after clinically apparent liver injury has not been reported. Also, there is no information on cross sensitivity to hepatic injury between gemcitabine and other antimetabolites or cytosine analogues such as cytarabine and capecitabine.
Drug Class: Antineoplastic Agents
Other Drugs in the Subclass, Pyrimidine Analogues: Azacitidine, Capecitabine, Cytarabine, Decitabine, Floxuridine, Fluorouracil, Trifluridine/Tipracil
CASE REPORTS
Case 1. Cholestatic liver injury arising during gemcitabine therapy.
[Modified from: Robinson K, Lambiase L, Li J, Monteiro C, Schiff M. Fatal cholestatic liver failure associated with gemcitabine therapy. Dig Dis Sci 2003; 48: 1804-8. PubMed Citation]
A 45 year old woman with metastatic breast adenocarcinoma was treated with eight cycles of taxotere, cyclophosphamide and daunorubicin and achieved remission. Liver tests were repeatedly normal before, during and after therapy. Two years later, she presented with symptoms of pain, nausea, vomiting and dehydration and was found to have elevations in liver tests (bilirubin 0.8 mg/dL, ALT 222 U/L, alkaline phosphatase 202 U/L). Tests for hepatitis B and C were negative and there were no abnormalities or clear evidence of metastatic disease on computerized tomography of the abdomen and chest. She, nevertheless, was started on gemcitabine and carboplatin for presumed recurrence of breast cancer. She improved somewhat but continued to have fever and poor appetite. Chemotherapy was continued. Twelve days after a fourth dose of gemcitabine and carboplatin, she was found to be jaundiced (bilirubin 6.4 mg/dL) and chemotherapy was stopped. Over the next two weeks, she developed worsening symptoms and required hospital admission for management. She had muscle wasting, peripheral edema, abdominal protuberance and tenderness, hepatomegaly and signs of hepatic failure (confusion and asterixis). Serum bilirubin was 14.7 mg/dL and other liver tests remained abnormal (Table). Serum ammonia was 49 µmol/L (normal <35) and INR was 1.4. Repeat CT scans showed marked ascites, pleural effusions, but no evidence of hepatic metastases. A liver biopsy showed patchy lobular necrosis, steatosis and marked cholestasis which was interpreted as compatible with drug induced liver injury. Her hepatic failure worsened and she died 2 days later, 5 days after hospitalization and 3 weeks after stopping gemcitabine and carboplatin.
Key Points
Laboratory Values
| Time After Starting | Days After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | Pre | 222 | 202 | 0.8 | Admission |
| 0 | 60 | 160 | 0.6 | Albumin 2.7g/dL | |
| Weekly infusions of gemcitabine and carboplatin started (x4) | |||||
| 5 days | 158 | 257 | 1.0 | ||
| 34 days | 0 | 39 | 213 | 6.4 | Chemotherapy stopped |
| 55 days | 21 days | 75 | 312 | 14.7 | Admission |
| 56 days | 22 days | 86 | 307 | 14.2 | INR 1.4 |
| 57 days | 23 days | 69 | 271 | 12.1 | Liver biopsy |
| 60 days | Death from hepatic failure | ||||
| Normal Values | <42 | <128 | <1.2 | ||
Comment
A relatively young woman with a history of breast cancer developed progressive liver disease with marked cholestasis and hepatic synthetic dysfunction concurrent with starting a regimen of carboplatin and gemcitabine for presumed recurrent, metastatic disease. Attribution of the injury to gemcitabine is difficult, as she was on other medications that are known to cause liver injury including anastrozole and carboplatin, which are perhaps more likely causes of the injury. Nevertheless, gemcitabine has been linked to cholestatic liver injury with modest serum aminotransferase and alkaline phosphatase elevations in patients with underlying liver disease. This patient had evidence of hepatic dysfunction before gemcitabine was started, and it well might have exacerbated the underlying condition. The course and outcome is somewhat reminiscent of fatty liver with lactic acidosis and hepatic dysfunction, which occurs with many nucleoside analogues in susceptible patients.
Case 2. Acute hepatocellular injury arising during gemcitabine therapy.
[Modified from: Saadati H, Peccerillo J, Kaley K, Schilsky ML, Saif MW. Gemcitabine-induced hepatitis in a pancreatic cancer patient receiving adjuvant therapy following metastasectomy. JOP 2009; 10: 573-5. PubMed Citation]
A 68 year old man with adenocarcinoma of the pancreas underwent a Whipple procedure, followed by three 28 day cycles of gemcitabine. He was then found to have hepatic metastasis and switched to the combination of gemcitabine and oxaliplatin which was continued for a year, at which time repeat imaging studies showed resolution of the hepatic masses. He was followed on no therapy and had no evidence of recurrence until 3 years later when a lung mass was detected and found to be a metastasis on resection. He was restarted on 28 day cycles of gemcitabine which were well tolerated until cycle six, when serum enzymes were found to be elevated and gemcitabine was held. He subsequently developed mild jaundice [bilirubin 3.7 mg/dL, ALT 1660 U/L, alkaline phosphatase 226 U/L] and he was admitted for evaluation. Tests for acute hepatitis were said to be negative and magnetic resonance imaging of the liver showed fatty infiltration, but no evidence of biliary obstruction or recurrence of hepatic metastases. He remained mildly jaundiced for several days, but complained of no symptoms and recovered rapidly and completely. In follow up, liver tests were normal. Gemcitabine was not restarted.
Key Points
| Medication: | Gemcitabine (six 28 day cycles; dose not given) |
|---|---|
| Pattern: | Hepatocellular (R=27) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | Approximately 5 months |
| Recovery: | Within 3 weeks |
| Other medications: | None mentioned |
Laboratory Values
* Several values estimated from Figure 1.
Comment
This patient with metastatic adenocarcinoma of the pancreas developed a mild, acute viral hepatitis-like syndrome after 5 cycles of gemcitabine. No other medications were mentioned. The report does not provide results of virologic and serological testing. Similar instances of acute hepatocellular injury with jaundice have not been reported with gemcitabine, and the possibility exists that the acute hepatitis was due to acute viral hepatitis (HEV or HCV are often difficult to diagnose in the acute setting).
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Gemcitabine – Generic, Gemzar®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
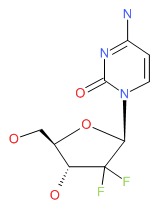
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Gemcitabine | 95058-81-4 | C9-H11-F2-N3-O4 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 09 March 2018
- Zimmerman HJ. Hepatic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity published in 1999; gemcitabine is not mentioned).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 541-68.(Review of hepatotoxicity of hepatotoxicity of anticancer agents; gemcitabine is listed as a frequent cause of mild to moderate aminotransferase elevations and linked to one case of acute liver failure).
- Chabner BA, Bertino J, Cleary J, Ortiz T, Lane A, Supko JG, Ryan DP. Gemcitabine. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1700-1.(Textbook of pharmacology and therapeutics; gemcitabine is a cytidine derivative that has become an important drug for patients with metastatic pancreatic, non-small cell lung, ovarian and bladder cancers).
- Anderson H, Lund B, Bach F, Thatcher N, Walling J, Hansen HH. Single-agent activity of weekly gemcitabine in advanced non-small-cell lung cancer: a phase II study. J Clin Oncol 1994; 12: 1821-6. [PubMed: 8083706](Among 79 patients with non-small-cell lung cancer treated with 368 courses of gemcitabine, 12% had ALT elevations above 5 times ULN, but all recovered without symptoms or jaundice).
- Abratt RP, Bezwoda WR, Falkson G, Goedhals L, Hacking D, Rugg TA. Efficacy and safety profile of gemcitabine in non-small-cell lung cancer: a phase II study. J Clin Oncol 1994; 12: 1535-40. [PubMed: 8040664](Among 76 patients with non-small cell lung cancer given gemcitabine, 73% had ALT elevations which rose above 5 times ULN in 13%).
- Tonato M, Mosconi AM, Martin C. Safety profile of gemcitabine. Anticancer Drugs 1995; 6 Suppl 6: 27-32. [PubMed: 8718422](Review of side effects of gemcitabine based upon results from 790 patients in pivotal studies; ALT elevations occurred in 68% of patients, but were above 5 times ULN in only 9.2% and the cause of discontinuation in only 0.5%).
- Martin C, Lund B, Anderson H, Thatcher N. Gemcitabine: once-weekly schedule active and better tolerated than twice-weekly schedule. Anticancer Drugs 1996; 7: 351-7. [PubMed: 8792011](Comparison of once vs twice weekly gemcitabine from 18 studies in various cancers; ALT elevations above 5 times ULN occurred in 9.2% of patients with once weekly versus 21.2% with twice weekly gemcitabine, but only 4 patients had to stop therapy because of liver test abnormalities; one patient ["who had a long history of chronic alcoholism"] died of liver failure).
- Carmichael J, Fink U, Russell RC, Spittle MF, Harris AL, Spiessi G, Blatter J. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer 1996; 73: 101-5. [PMC free article: PMC2074288] [PubMed: 8554969](Among 34 patients with advanced pancreatic carcinoma treated with gemcitabine, ALT elevations occurred in 90% of patients, but were above 5 times ULN in only ~15% and all were "reversible").
- Kaufmann M, von Minckwitz G. Gemcitabine in ovarian cancer: an overview of safety and efficacy. Eur J Cancer 1997 Jan; 33 Suppl 1: S31-3. [PubMed: 9166098](Review of status of gemcitabine in ovarian carcinoma; no mention of hepatotoxicity).
- Green MR. Gemcitabine safety overview. Semin Oncol 1996; 23(5 Suppl 10): 32-5. [PubMed: 8893879](Review of side effects of gemcitabine from 790 patients in 18 studies in various cancers; "mild transaminase increases were common but were rarely dose limiting").
- Cortes-Funes H, Martin C, Abratt R, Lund B. Safety profile of gemcitabine, a novel anticancer agent, in non-small cell lung cancer. Anticancer Drugs 1997; 8: 582-7. [PubMed: 9300572](Analysis of side effects of gemcitabine based upon results from 360 patients with non-small cell lung cancer; "transient asymptomatic and rapidly reversible" elevations of ALT occurred in 70% of patients and were above 5 times ULN in 12%).
- Yokoyama A, Nakai Y, Yoneda S, Kurita Y, Niitani H. Activity of gemcitabine in the treatment of patients with non-small cell lung cancer: a multicenter phase II study. Anticancer Drugs 1997; 8: 574-81. [PubMed: 9300571](Among 67 patients with non-small cell lung cancer treated with 2 to 20 courses of gemcitabine, ALT or AST elevations occurred in 36% of patients; no mention of clinically apparent liver injury).
- Zatloukal P, Kanitz E, Magyar P, Jassem J, Krzakowski M, Pawlicki M, Petruzelka L, et al. Gemcitabine in locally advanced and metastatic non-small cell lung cancer: the Central European phase II study. Lung Cancer 1998; 22: 243-50. [PubMed: 10048477](Among 80 patients with advanced non-small cell lung cancer treated with 1 to 9 cycles of gemcitabine, ALT elevations occurred in 28% but were above 5 times ULN in only 1.3%, and were not associated with clinically apparent liver injury).
- Zinzani PL, Magagnoli M, Bendandi M, Orcioni GF, Gherlinzoni F, Albertini P, Pileri SA, et al. Therapy with gemcitabine in pretreated peripheral T-cell lymphoma patients. Ann Oncol 1998; 9: 1351-3. [PubMed: 9932168](Among 13 patients with lymphoma treated with gemcitabine, one had transient ALT elevations).
- Lorusso V, Pollera CF, Antimi M, Luporini G, Gridelli C, Frassineti GL, Oliva C, et al. A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Italian Co-operative Group on Bladder Cancer. Eur J Cancer 1998; 34: 1208-12. [PubMed: 9849481](Among 35 patients with urinary tract carcinoma treated with gemcitabine, ALT or AST elevations above 5 times ULN occurred in 11%).
- Aapro MS, Martin C, Hatty S. Gemcitabine.a safety review. Anticancer Drugs 1998; 9: 191-201. [PubMed: 9625429](Review of safety of gemcitabine based upon results from 22 studies in 979 patients with various cancers; 42% of courses were associated with ALT elevations, but they were usually mild and transient, being above 5 times ULN in only 9.6% and resulting in drug discontinuation in only 0.5% of patients).
- Dobbie M, Hofer S, Oberholzer M, Herrmann R. Veno-occlusive disease of the liver induced by gemcitabine. Ann Oncol 1998; 9: 681. [PubMed: 9681086](48 year old woman with chronic hepatitis C and non-small cell lung cancer developed sinusoidal obstruction syndrome after 4 cycles of gemcitabine [bilirubin 5 times ULN, ALT 10 times ULN], with progressive liver failure and death within 8 days, autopsy showing sinusoidal obstruction).
- Oettle H, Pelzer U, Hochmuth K, Diebold T, Langrehr J, Schmidt CA, Arning M, et al. Phase I trial of gemcitabine (Gemzar), 24 h infusion 5-fluorouracil and folinic acid in patients with inoperable pancreatic cancer. Anticancer Drugs 1999; 10: 699-704. [PubMed: 10573201](Among 16 patients with advanced pancreatic cancer treated with 5FU, folinic acid and gemcitabine, hepatotoxicity was dose limiting for 5FU and one patient treated with the highest dose died of hepatic failure).
- Coeman DC, Verbeken EK, Nackaerts KL, Demedts MG, Vansteenkiste JF. A fatal case of cholestatic liver failure probably related to gemcitabine. Ann Oncol 2000; 11: 1503. [PubMed: 11142494](73 year old man with undifferentiated metastatic carcinoma developed progressive jaundice after the 6th 28 day cycle of gemcitabine, leading to multiorgan failure and death, autopsy showing bland centrolobular necrosis with cholestasis).
- Savage DG, Rule SA, Tighe M, Garrett TJ, Oster MW, Lee RT, Ruiz J, et al. Gemcitabine for relapsed or resistant lymphoma. Ann Oncol 2000; 11: 595-7. [PubMed: 10907954](Study of 15 patients with relapsed or resistant lymphoma treated with gemcitabine makes no mention of ALT elevations or hepatotoxicity).
- Samlowski WE, Gundacker H, Kuebler JP, Giguere JK, Mills GM, Schuller DE, Ensley JF. Evaluation of gemcitabine in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: a Southwest Oncology Group phase II study. Invest New Drugs 2001; 19: 311-5. [PubMed: 11561690](Among 26 patients with advanced head and neck cancer, one died of liver failure after the initial infusion, thought to be due to progressively enlarging liver metastases).
- Spielmann M, Llombart-Cussac A, Kalla S, Espié, Namer M, Ferrero JM, Diés V, et al. Single-agent gemcitabine is active in previously treated metastatic breast cancer. Oncology 2001; 60: 303-7. [PubMed: 11408796](Among 47 patients with metastatic breast cancer treated with gemcitabine, one patient with severe liver involvement at entry died of liver failure 5 days after the initial infusion; no other mention of ALT elevations or hepatotoxicity).
- Blackstein M, Vogel CL, Ambinder R, Cowan J, Iglesias J, Melemed A. Gemcitabine as first-line therapy in patients with metastatic breast cancer: a phase II trial. Oncology 2002; 62: 2-8. [PubMed: 11810037](Among 34 patients with metastatic breast cancer treated with gemcitabine, 65% had ALT elevations which were above 5 times ULN in 12%).
- Ikeda M, Okada S, Tokuuye K, Ueno H, Okusaka T. A phase I trial of weekly gemcitabine and concurrent radiotherapy in patients with locally advanced pancreatic cancer. Br J Cancer 2002; 86: 1551-4. [PMC free article: PMC2746589] [PubMed: 12085203](Among 15 patients with locally advanced pancreatic cancer given gemcitabine in increasing doses, ALT elevations above 5 times ULN occurred in 33% and required dose modification in 2 patients given the highest tolerated doses).
- Cheong K, Li J, Karapetis CS. Gemcitabine and reactivation of hepatitis B. Med Oncol 2003; 20: 385-8. [PubMed: 14716036](70 year old woman with HBsAg and an undifferentiated carcinoma developed rise in HBV DNA [2,700 to 160,000 copies/mL] and ALT [41 to 255 U/L], without symptoms or jaundice after three 28 day cycles of gemcitabine [without other agents], values returning to baseline within 2 months of stopping).
- Robinson K, Lambiase L, Li J, Monteiro C, Schiff M. Fatal cholestatic liver failure associated with gemcitabine therapy. Dig Dis Sci 2003; 48: 1804-8. [PubMed: 14561005](45 year old woman with breast cancer with liver metastases and ALT elevations developed jaundice soon after starting gemcitabine [bilirubin 6.4 rising to 14.7 mg/dL, ALT 222 U/L, Alk P 202 U/L], with hepatic failure and death; liver biopsy showing fat, cholestasis and patchy hepatocellular necrosis: Case 1).
- Modi S, Seidman AD. Single-agent gemcitabine in the treatment of advanced breast cancer. Clin Breast Cancer 2004; 4 Suppl 3: S101-6. [PubMed: 14754466](Review of role of gemcitabine as a single agent in advanced breast cancer, summarizing 10 phase II trials; in one study, ALT elevations above 5 times ULN occurred in 18% of patients, but hepatotoxicity was not otherwise discussed).
- Kosmidis PA, Kalofonos HP, Christodoulou C, Syrigos K, Makatsoris T, Skarlos D, Bakogiannis C, et al. Paclitaxel and gemcitabine versus carboplatin and gemcitabine in patients with advanced non-small-cell lung cancer. A phase III study of the Hellenic Cooperative Oncology Group. Ann Oncol 2008; 19: 115-22. [PubMed: 17938425](Among 452 patients with advanced non-small cell lung cancer randomized to receive gemcitabine with either paclitaxel or carboplatin, ALT or AST elevations above 5 times ULN occurred in 0.9% vs 1.2% of patients, but other toxicities were more common with the carboplatin than paclitaxel regimen).
- Matsuda M, Watanabe G, Mine S, Hashimoto M. [Fatal liver failure associated with gemcitabine hydrochloride therapy]. Gan To Kagaku Ryoho 2008; 35: 157-9. Japanese. [PubMed: 18195549](79 year old man with chronic hepatitis C and pancreatic cancer developed jaundice after radiation therapy and the 6th cycle of gemcitabine [bilirubin 4.0 rising to 9.2 mg/dL, ALT 77 U/L, Alk P 560 U/L], with progression to liver failure and death; autopsy showing portal vein invasion by cancer and extensive centrolobular necrosis).
- Saadati H, Peccerillo J, Kaley K, Schilsky ML, Saif MW. Gemcitabine-induced hepatitis in a pancreatic cancer patient receiving adjuvant therapy following metastasectomy. JOP 2009; 10: 573-5. [PubMed: 19734642](68 year old man with advanced, metastatic pancreatic cancer developed dark urine and liver test abnormalities 5 cycles of gemcitabine [bilirubin 3.7 mg/dL, ALT 1660 U/L, Alk P 226 U/L], resolving within 7 weeks of stopping: Case 2).
- Tanaka H, Takamori H, Eto S, Ozaki N, Akaboshi S, Nakahara O, Ida S, et al. [Acute liver injury with hepatic encephalopathy associated with gemcitabine administration for adjuvant chemotherapy in an HBV carrier with pancreatic cancer]. Gan To Kagaku Ryoho 2010; 37: 1783-6. Japanese. [PubMed: 20841947](75 year old HBsAg positive woman with pancreatic cancer developed jaundice after a 6th weekly dose of gemcitabine [bilirubin 3.7 mg/dL, ALT 37 U/L, Alk P 471 U/L, INR 1.9, ammonia 72 rising to 300 µg/dL], without change in hepatitis B markers [HBV DNA undetectable], resolving within 4 weeks of stopping therapy).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 2 attributed to antineoplastic agents, 1 to melphalan and 1 to gemtuzumab, but none gemcitabine or other antimetabolites).
- García Del-Muro X, López-Pousa A, Maurel J, Martín J, Martínez-Trufero J, Casado A, Gómez-España A, et al.; Spanish Group for Research on Sarcomas. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011; 29: 2528-33. [PubMed: 21606430](Among 109 patients with soft tissue sarcoma treated with 386 cycles of dacarbazine with or without gemcitabine, overall survival was better with the combination; no mention of ALT elevations or hepatotoxicity).
- Choi JG, Seo JH, Oh SC, Choi CW, Kim JS. A phase II trial of gemcitabine plus capecitabine for patients with advanced pancreatic cancer. Cancer Res Treat 2012; 44: 127-32. [PMC free article: PMC3394862] [PubMed: 22802751](Among 50 patients with advanced pancreatic cancer treated with the combination of gemcitabine and capecitabine, median overall survival was 10 months; no mention of ALT elevations or hepatotoxicity).
- Ioka T, Katayama K, Tanaka S, Takakura R, Ashida R, Kobayashi N, Taniai H. Safety and effectiveness of gemcitabine in 855 patients with pancreatic cancer under Japanese clinical practice based on post-marketing surveillance in Japan. Jpn J Clin Oncol 2013; 43: 139-45. [PubMed: 23275642](Among 890 patients with advanced pancreatic cancer treated with gemcitabine and reported to a Japanese registry, hepatic adverse events were reported in 1.3% of patients, but none were graded as severe).
- Joerger M, Huitema AD, Koeberle D, Rosing H, Beijnen JH, Hitz F, Cerny T, et al. Safety and pharmacology of gemcitabine and capecitabine in patients with advanced pancreatico-biliary cancer and hepatic dysfunction. Cancer Chemother Pharmacol 2014; 73: 113-24. [PubMed: 24166106](Among 12 patients with advanced pancreatic or biliary cancer and varying degrees of liver dysfunction, the toxicity of gemcitabine [largely hematologic] did not correlate with degree of liver dysfunction).
- Yang ZY, Yuan JQ, Di MY, Zheng DY, Chen JZ, Ding H, Wu XY, et al. Gemcitabine plus erlotinib for advanced pancreatic cancer: a systematic review with meta-analysis. PLoS One 2013; 8: e57528. [PMC free article: PMC3589410] [PubMed: 23472089](Review of literature on efficacy and safety of the combination of erlotinib and gemcitabine for advanced pancreatic cancer; the addition of erlotinib was associated with a prolongation of survival by 0.3 months and the adverse event rate was high "but not surprising"; no mention of hepatotoxicity).
- Fuchs CS, Azevedo S, Okusaka T, Van Laethem JL, Lipton LR, Riess H, Szczylik C, et al. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol 2015; 26 :921-7. [PMC free article: PMC4804122] [PubMed: 25609246](Among 800 patients with metastatic pancreatic cancer treated with gemcitabine with or without ganitumab [monoclonal anti-IGF1R], overall survival was the same in all groups, and ALT elevations arose in 16% but were above 5 times ULN in less than 1%).
- O'Neil BH, Scott AJ, Ma WW, Cohen SJ, Leichman L, Aisner DL, Menter AR, et al. A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. Ann Oncol 2015; 26: 1923-9. [PMC free article: PMC4551155] [PubMed: 26091808](Among 160 patients with metastatic pancreatic cancer treated with gemcitabine with or without rigosertib [a multikinase inhibitor], overall survival was the same in both groups and ALT elevations arose in 13% of patients and were above 5 times ULN in 1%).
- Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, et al.; JASPAC 01 Study Group. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016; 388: 248-57. [PubMed: 27265347](Among 377 Japanese patients with pancreatic cancer given adjuvant chemotherapy with gemcitabine or "S-1", ALT elevations arose in 78% of patients and were above 5 times ULN in 4%).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. PubMed Citation. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 [5.5%] were attributed to antineoplastic agents, but none were linked to use of gemcitabine).
- Schultheis B, Reuter D, Ebert MP, Siveke J, Kerkhoff A, Berdel WE, Hofheinz R, et al. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen in KRAS wildtype patients with locally advanced or metastatic pancreatic cancer: a multicenter, randomized phase IIb study. Ann Oncol 2017; 28: 2429-35. [PubMed: 28961832](Among 192 patients with pancreatic cancer treated with gemcitabine with or without nimotuzumab [anti-EGFR], median overall survival was greater with the combination [8.6 vs 5.1 months] while adverse event rates were similar; ALT elevations and hepatotoxicity not mentioned).
- Benson AB 3rd, Wainberg ZA, Hecht JR, Vyushkov D, Dong H, Bendell J, Kudrik F. A Phase II randomized, double-blind, placebo-controlled study of simtuzumab or placebo in combination with gemcitabine for the first-line treatment of pancreatic adenocarcinoma. Oncologist 2017; 22: 241-e15. [PMC free article: PMC5344644] [PubMed: 28246206](Among 240 patients with metastatic pancreatic cancer treated with gemcitabine with or without simtuzumab [monoclonal anti-LOXL1], overall survival was similar in both groups as were adverse event rates with ALT elevations in 89% and 82% of subjects).
- Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, et al.; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017; 389: 1011-24. [PubMed: 28129987](Among 730 patients with resected pancreatic cancer given adjuvant chemotherapy with gemicitabine with or without capecitabine, overall survival was 25.5 vs 28.0 months while adverse event rates were only slightly higher with the combination; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- A Severe Case of Drug-Induced Liver Injury after Gemcitabine Administration: A Highly Probable Causality Grading as Assessed by the Updated RUCAM Diagnostic Scoring System.[Case Reports Hepatol. 2020]A Severe Case of Drug-Induced Liver Injury after Gemcitabine Administration: A Highly Probable Causality Grading as Assessed by the Updated RUCAM Diagnostic Scoring System.Mascherona I, Maggioli C, Biggiogero M, Mora O, Marelli L. Case Reports Hepatol. 2020; 2020:8812983. Epub 2020 Oct 1.
- Review Fluorouracil.[LiverTox: Clinical and Researc...]Review Fluorouracil.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Decitabine.[LiverTox: Clinical and Researc...]Review Decitabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Capecitabine.[LiverTox: Clinical and Researc...]Review Capecitabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Cytarabine.[LiverTox: Clinical and Researc...]Review Cytarabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Gemcitabine - LiverToxGemcitabine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...