NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Gemfibrozil is a fibric acid derivative used in the therapy of hypertriglyceridemia and dyslipidemia. Gemfibrozil therapy is associated with mild and transient serum aminotransferase elevations and with rare instances of acute liver injury.
Background
Gemfibrozil (jem fye' broe zil) is a fibric acid derivative and lipid lowering agent. The lipid lowering activity of gemfibrozil is probably mediated by its interactions with the peroxisome proliferator activated receptor alpha (PPARα), which regulates gene expression of enzymes involved in fatty acid oxidation. Gemfibrozil increases lipoprotein lipase levels, which enhance clearance of triglyceride rich lipoproteins. Gemfibrozil was approved for use in the United States in 1981 and it is still widely used with more than 5 million prescritions filled yearly. Gemfibrozil is recommended for therapy of hypertriglyceridemia (Fredrickson type IV and V hyperlipidemia) and hypercholesterolemia (type IIb). Gemfibrozil therapy has been shown to lower rates of myocardial infarction and stroke, most likely as a result of its effects on HDL cholesterol and triglyceride levels. Gemfibrozil is available in multiple generic forms and under the brand name of Lopid as tablets of 600 mg. The recommended dosage is 600 mg twice daily before morning and evening meals. Common side effects of gemfibrozil include gastrointestinal upset, diarrhea, constipation and fatigue. Fibrates have multiple drug interactions requiring careful review and use.
Hepatotoxicity
Mild, transient serum aminotransferase elevations develop in approximately 20% of patients receiving gemfibrozil, but values above 3 times normal in 5% or less. These abnormalities are usually asymptomatic and transient, resolving even with continuation. However, there have also been rare reports of clinically apparent liver injury in patients on long term gemfibrozil. The clinical presentation was highly variable. The onset of injury varied from a few weeks to several years after starting the medication and the pattern of serum enzyme elevations ranged from hepatocellular (Case 1) to mixed to cholestatic. Cases have not been associated with signs of immunoallergic (fever, rash, eosinophilia) or autoimmune hepatitis and recovery has been prompt and complete with stopping therapy.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of hepatotoxicity of the gemfibrozil is not known, but is likely due to an immunologic response to an intermediate of its metabolism.
Outcome and Management
The few published instances of clinically apparent hepatotoxicity due to gemfibrozil have been mild and self-limited. There have been no published reports of acute liver failure, chronic hepatitis or vanishing bile duct syndrome related to gemfibrozil. It is unclear whether liver injury is a class effect of the fibrates. There is little evidence for cross sensitivity to hepatotoxicity among the various fibrates.
Drug Class: Antilipemic Agents, Fibrates
CASE REPORT
Case 1. Mild acute hepatitis due to gemfibrozil.
[Modified from: Domínguez Tordera P, Comellas Alabern JF, Ronda Rivero F. Gemfibrozil hepatotoxicity: a case report. Int J Clin Pharm 2011; 33: 730-2. PubMed Citation]
A 55 year old woman developed nausea and fatigue one month after starting gemfibrozil (600 mg daily). Several months before this she had been involved in a motor vehicle accident and was admitted to the hospital for management of thoracic and ankle trauma. At that time, all liver tests were normal and an ultrasound of the abdomen (done to assess trauma) showed small gallstones, but no abnormalities of intra- or extra-hepatic bile ducts. She was treated for trauma and fractures and was started on enoxaparin, esomeprazole, venlafaxine and metamizol (an analgesic and antipyretic not available in the United States). During follow up after the admission, she was found to have hypertriglyceridemia (239 mg/dL), but normal cholesterol levels and was started on gemfibrozil, which she had not taken previously. She had no history of liver disease, alcohol use, or known risk factors for viral hepatitis. She denied rash, fever and abdominal pain. Blood tests at the time of onset of fatigue showed marked elevations in serum aminotransferase levels (ALT 1142 U/L, AST 656 U/L), modest increase in alkaline phosphatase (331 U/L) and GGT (420 U/L), and a total serum bilirubin of 2.7 mg/dL (2.2 mg/dL direct). Tests for hepatitis A, B and C were negative. Tests for autoantibodies were not mentioned. Abdominal ultrasound showed the previously described gallstones, but no evidence of biliary obstruction. Gemfibrozil was discontinued, but her other medications were not. Serum enzyme abnormalities rapidly improved and were largely normal 2 weeks later (Table).
Key Points
| Medication: | Gemfibrozil (600 mg daily) |
| Pattern: | Hepatocellular (R=12.4) |
| Severity: | 2+ (mild jaundice) |
| Latency: | 1 month |
| Recovery: | 2 months |
| Other medications: | Enoxaparin, esomeprazole, venlafaxine, metamizol |
Laboratory Values
Comment
Acute hepatocellular injury arose within a month of starting gemfibrozil therapy. The patient was taking several other drugs that might cause liver injury (enoxaparin, venlafaxine and even esomeprazole), but these were continued and only the gemfirbrozil was held. She improved rapidly which was evident within 2 days and nearly complete within 2 weeks. The acute hepatocellular injury with mild jaundice and rapid recovery (<1 month) on stopping the drug are typical of gemfibrozil induced liver injury.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Gemfibrozil – Generic, Lopid®
DRUG CLASS
Antilipemic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
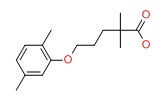
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Gemfibrozil | 25812-30-0 | C15-H22-O3 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 24 January 2017
- Zimmerman HJ. Drugs used in the treatment of hypercholesterolemia and hyperlipidemia. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 660-2.(Expert review of hepatotoxicity of lipid lowering agents including clofibrate, fenofibrate and gemfibrozil, all three of which can lead to mild-to-moderate serum aminotransferase elevations, and which have been associated with rare instances of clinically apparent hepatic injury).
- De Maurzio DH, Navarro VJ. Fibrates. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 527.(Review of hepatotoxicity of fibrates; transient ALT or AST elevations during therapy are common and rare instances of acute and chronic hepatic injury due to fibrates have been reported, with variable patterns of injury and times of onset).
- Bersot TP. Drug therapy for hypercholesterolemia and dyslipidemia. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 877-908.(Textbook of pharmacology and therapeutics).
- De La Iglesia FA, Lewis JE, Buchanan AR, Marcus EL, McMahon G. Light and electron microscopy in hyperlipoproteinemic patients under long-term gemfibrozil treatment. Atherosclerosis 1982; 43: 19-37. PubMed Citation. [PubMed: 6807326](Detailed analysis of light and electron microscopic changes in liver biopsies from 9 patients without liver test abnormalities on successful long term [18-27 months] therapy with gemfibrozil; mild fatty change was found, but there was minimal to no abnormalities of peroxisomes and mitochondria such as have been reported in rodents given gemfibrozil).
- Pickering JE. Clinical results with gemfibrozil. Am J Cardiol 1983; 52: 39B-40B. [PubMed: 6351579](Review of clinical trials of gemfibrozil; most common side effects were gastrointestinal upset and liver test abnormalities occurred in <3% of patients: Abstract only).
- Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987; 317: 1237-45. [PubMed: 3313041](In a controlled trial of gemfibrozil vs placebo for up to 5 years in 4081 men with dyslipidemia, "no differences between the two treatment groups were observed in...laboratory multichannel analyses").
- Athyros VG, Athyros VG, Papageorgiou AA, Hatzikonstandinou HA, Didangelos TP, Carina MV, Kranitsas DF, et al. Safety and efficacy of long-term statin-fibrate combinations in patients with refractory familial combined hyperlipidemia. Am J Cardiol 1997; 80: 608-13. [PubMed: 9294990](389 patients treated with statin and fibrate combination for average of 2.5 years; 1.3% stopped because of ALT above 3 times normal, all resolving within 4 weeks; no hepatitis or jaundice reported).
- Frick MH, Syvänne M, Nieminen MS, Kauma H, Majahalme S, Virtanen V, Kesäniemi YA, et al. Prevention of the angiographic progression of coronary and vein-graft atherosclerosis by gemfibrozil after coronary bypass surgery in men with low levels of HDL cholesterol. Lopid Coronary Angiography Trial (LOCAT) Study Group. Circulation 1997; 96: 2137-43. [PubMed: 9337181](Controlled trial of gemfibrozil vs placebo in 395 men after coronary bypass surgery; "significant" liver enzyme elevations occurred in 9 gemfibrozil [5%], but no placebo recipient; no details provided).
- Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999; 341: 410-8. [PubMed: 10438259](Controlled trial of gemfibrozil vs placebo for an average of 5 years in 2531 men with coronary artery disease, the numbers of patients with AST elevations "did not differ significantly between the groups").
- Bustamante Balén M, Plumé Gimeno G, Bau González I, Berenguer Lapuerta J. Acute hepatitis caused by gemfibrozil. Gastroenterol Hepatol 1998; 21: 419-20. [PubMed: 9844285](69 year old woman developed dark urine after 2 years of therapy with gemfibrozil [bilirubin 3.1 mg/dL, ALT 1931 U/L], falling to normal within one month of stopping).
- de Diego Lorenzo A, Catalina V, García Sánchez A, Escudero M, Cos E, Clemente G. Cholestatic hepatitis caused by gemfibrozil. Rev Esp Enferm Dig 2001; 93; 610-1. [PubMed: 11767440](67 year old man developed jaundice 2 years after starting gemfibrozil [bilirubin 8.2 mg/dL, ALT 524 U/L, Alk P 551 U/L], resolving rapidly with stopping drug).
- Alsheikh-Ali AA, Kuvin JT, Karas RH. Risk of adverse events with fibrates. Am J Cardiol 2004; 94: 935-8. [PubMed: 15464682](Review of adverse event reports to FDA for gemfibrozil and fenofibrate from 1999-2003; hepatotoxicity report rates were 13 per million for gemfibrozil vs 14.6 per million for fenofibrate).
- Grubisić-Cabo F, Vrdoljak E. Drug-induced hepatitis in a patient with malignant melanoma treated with interferon alfa 2b adjuvantly who had been administered gemfibrozil in therapy. Med Oncol 2006; 23: 121-4. [PubMed: 16645237](61 year old with melanoma was treated with interferon alfa [15 mu thrice weekly] for 3 months and then developed rises in ALT [34 to 708 U/L] and bilirubin [0.7 to 1.3 mg/dL] within a few weeks of starting gemfibrozil, resolving within a few weeks of stopping both).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, et al.; Spanish Group for the Study of Drug-Induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005; 129: 512-21. [PubMed: 16083708](Analysis of 461 cases of drug induced liver disease 1984 to 2004 in Spanish Registry; 4 cases were attributed to fibrates).
- Andrade RJ, Lucena MI, Kaplowitz N, García-Muņoz B, Borraz Y, Pachkoria K, García-Cortés M, et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology 2006; 44: 1581-8. [PubMed: 17133470](28 of 493 [5.7%] cases of drug induced liver disease were found to have evidence of chronic injury; 2 chronic cholestatic cases were attributed, at least in part, to fibrates; gemfibrozil/lovastatin and fenofibrate/raloxifen).
- Akoglu H, Yilmaz R, Kirkpantur A, Arici M Altun B, Turgan C. Combined organ failure with combination antihyperlipidemic treatment: a case of hepatic injury and acute renal failure. Ann Pharmacother 2007; 41: 143-7. [PubMed: 17148651](56 year old woman developed fatigue and red urine 1 month after starting combination of fluvastatin and gemfibrozil [bilirubin normal, ALT 2100 U/L, Alk P normal, creatinine 1.8 mg/dL, CPK 45,758 U/L, and myoglobin >3000 ng/dL], resolving rapidly upon stopping both drugs).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, 1 case attributed to fenofibrate; none to clofibrate or gemfibrozil).
- Domínguez Tordera P, Comellas Alabern JF, Ronda Rivero F. Gemfibrozil hepatotoxicity: a case report. Int J Clin Pharm 2011; 33: 730-2. [PubMed: 21881934](55 year old woman developed fatigue 1 month after starting gemfibrozil for hypertriglyceridemia [bilirubin 2.7 mg/dL, ALT 1142 U/L, Alk P 331 U/L], resolving within 2 weeks of stopping: Case 1).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to a fibrate).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to a fibrate).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature on drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to a fibrate).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 4 cases [0.5%] were attributed to fibrates, all to fenofibrate).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Clofibrate.[LiverTox: Clinical and Researc...]Review Clofibrate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Fenofibrate.[LiverTox: Clinical and Researc...]Review Fenofibrate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Comparison in patients with type 2 diabetes of fibric acid versus hepatic hydroxymethyl glutaryl-coenzyme a reductase inhibitor treatment of combined dyslipidemia.[Metabolism. 2002]Comparison in patients with type 2 diabetes of fibric acid versus hepatic hydroxymethyl glutaryl-coenzyme a reductase inhibitor treatment of combined dyslipidemia.McLaughlin T, Abbasi F, Lamendola C, Leary E, Reaven GM. Metabolism. 2002 Oct; 51(10):1355-9.
- Lipid-lowering effects of simvastatin and gemfibrozil in CAPD patients: a prospective cross-over study.[Adv Perit Dial. 1996]Lipid-lowering effects of simvastatin and gemfibrozil in CAPD patients: a prospective cross-over study.Akçiçek F, Ok E, Duman S, Kürsad S, Unsal A, Alev M, Atabay G, Basçi A. Adv Perit Dial. 1996; 12:261-5.
- Review Fibrates.[LiverTox: Clinical and Researc...]Review Fibrates.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Gemfibrozil - LiverToxGemfibrozil - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...