NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Cytarabine is a cytosine analogue and antineoplastic agent used largely in the therapy of acute leukemia. Cytarabine is associated with a low rate of transient serum enzyme and bilirubin elevations during therapy, but has only rarely been implicated in cases of clinically apparent acute liver injury with jaundice.
Background
Cytarabine (sye tar' a been) is an nucleoside analogue (cytosine arabinoside: ara-C) which is converted intracellularly to a triphosphate, which competes with cytosine triphosphate for incorporation into RNA and DNA and acts as an inhibitor of RNA and DNA polymerase, thus blocking DNA synthesis and cell division. Cytarabine has potent activity in acute leukemia and was approved for use in the United States in 1969 and is still widely used. Current indications include initial, consolidation and maintenance therapy of acute myelogenous and other acute leukemias. Cytarabine is available in vials of 100, 500, 1000 and 2000 mg (20 mg/mL) for intravenous or intrathecal infusion generically and under the brand name Cytosar-U. Liposomal sustained release formulations are also available for intrathecal administration (DepoCyt). The dose regimen of cytarabine varies by body surface area and indication. A typical induction dose is 100 mg per meter squared by continuous intravenous infusion on days 1 to 7. Common side effects include bone marrow suppression, nausea, vomiting, oral or anal ulcers, abdominal pain, myalgias, bone pain, chest pain, conjunctivitis headache, fatigue, fever, rash and pruritus.
Hepatotoxicity
Serum aminotransferase elevations occur in 5% to 10% of patients on conventional doses of cytarabine and a greater proportion (9% to 75%) at higher doses. However, the serum enzyme elevations are rarely associated with symptoms and are generally self-limited and resolve rapidly, rarely requiring dose modification. Cases of clinically apparent liver injury attributed to cytarabine have been reported but are uncommon. The time to onset was usually within the first few cycles of therapy, and the pattern of serum enzyme elevations ranged from cholestatic to hepatocellular. Immunoallergic and autoimmune features were generally not present. Antineoplastic regimens, including cytarabine, have been implicated in cases of sinusoidal obstruction syndrome and peliosis, but the role of cytarabine in these reactions was unclear. Many examples of liver injury attributed to cytarabine in the literature were typical of jaundice of sepsis rather than acute hepatocellular or cholestatic injury, although high doses of cytarabine may cause hyperbilirubinemia independent of hepatic injury.
Likelihood score: C (probable cause of clinically apparent liver injury).
Mechanism of Injury
While hepatotoxicity from cytarabine may be rare, it is likely due to direct toxicity to hepatocytes. Cytarabine is metabolized in the liver via the cytochrome P450 system and production of a toxic or immunogenic intermediate may trigger liver injury.
Outcome and Management
The severity of the liver injury linked to cytarabine therapy is usually mild and self-limited. Cytarabine has been linked to cases of acute liver failure but the relationship to cytarabine has not always been clear. Cytarabine has not been linked chronic hepatitis or vanishing bile duct syndrome. The product label for cytarabine recommends "periodic checks" of bone marrow, renal and kidney function" during therapy. There is no information on cross sensitivity to hepatic injury between cytarabine and other pyrimidine analogues.
Drug Class: Antineoplastic Agents
Other Drugs in the Subclass, Pyrimidine Analogues: Azacitidine, Capecitabine, Decitabine, Floxuridine, Fluorouracil, Gemcitabine, Trifluridine/Tipracil
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cytarabine – Generic, Cytosar-U®, DepoCyt®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Cytarabine | 147-94-4 | C9-H13-N3-O5 |
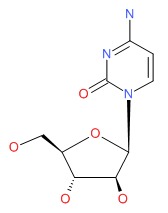 |
ANNOTATED BIBLIOGRAPHY
References updated: 03 November 2017
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; mentions that the hepatotoxic effects of cytarabine appear to be dose related, AST elevations occurring in 5-10% of patients at lowest and 22-75% at highest doses, which are also associated with occasional cases of clinically apparent cholestatic or hepatocellular injury).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68. (Review of hepatotoxicity of cancer chemotherapeutic agents; mentions that cytarabine has been implicated in.case reports of cholestatic hepatitis and peliosis).
- Chabner BA, Bertino J, Clearly J, Ortiz T, Lane A, Supko JG, Ryan DP. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1315-404.(Textbook of pharmacology and therapeutics; states that cytarabine is the most effective agent in therapy of acute myelogenous leukemia [AML]).
- Ellison RR, Holland JF, Weil M, Jacquillat C, Boiron M, Bernard J, Sawitsky A, et al. Arabinosyl cytosine: a useful agent in the treatment of acute leukemia in adults. Blood 1968; 32: 507-23. [PubMed: 4879053](In an early dose regimen finding study, serum bilirubin elevations occurred in 10-15% of patients treated with cytarabine, but were largely due to sepsis or hemolytic reactions; there were also "minor variations" in AST and Alk P that resolved with stopping therapy and did not recur on restarting).
- Bodey GP, Freireich EJ, Monto RW, Hewlett JS. Cytosine arabinoside(NSC-63878) therapy for acute leukemia in adults. Cancer Chemother Rep 1969; 53: 59-66. [PubMed: 5772656](Among patients with refractory leukemia who received 5 day courses of cytarabine, elevations in AST occurred during 9 of 42 courses, but all resolved rapidly and no patient developed symptoms or jaundice).
- Cytarabine (Cytosar). Clin Pharmacol Ther 1970; 11: 155-60. [PubMed: 5262667](Initial product labeling of cytarabine shortly after its initial approval for use in the US; hepatic dysfunction is said to occur during treatment in 7.1% of adults and 5.5% of children; cytarabine "is suspected of being hepatotoxic, but this is unproven").
- Wang JJ, Selawry OS, Vietti TJ, Bodey GP Sr. Prolonged infusion of arabinosyl cytosine in childhood leukemia. Cancer 1970; 25: 1-6. [PubMed: 5262259](46 children with refractory acute leukemia were treated with 5 day courses of intravenous cytarabine [1.2 g/m2 daily] every two weeks; side effects included myelosuppression, nausea, vomiting, anorexia, diarrhea, phlebitis and mild elevations in aminotransferase levels in 2 patients [~5%]).
- Burke PJ, Owens AH Jr, Colsky J, Shnider BI, Edmonson JH, Schilling A, Brodovsky HS, Wallace HJ Jr, Hall TC. A clinical evaluation of a prolonged schedule of cytosine arabinoside (NSC63878). Cancer Res 1970; 30: 1512-5. [PubMed: 5426952](Among 85 patients with metastatic cancer treated with cytarabine in repeated 10 day courses, "moderate and transient" elevations in liver tests occurred in some patients).
- Traggis DG, Dohlwitz A, Das L, Jaffe N, Moloney WC, Hall TC. Cytosine arabinoside in acute leukemia of childhood. Cancer 1971; 28: 815-8. [PubMed: 5286443](Among 74 children with acute leukemia treated with intravenous cytarabine [25 mg/kg] twice weekly, 4 developed jaundice, but all resolved with dose modification; few details given).
- Hryniuk W, Foerster J, Shojania M, Chow A. Cytarabine for herpesvirus infections. JAMA 1972; 219: 715-8. [PubMed: 4333390](Among 7 patients with severe herpesvirus infections [varicella-zoster and herpes simplex] treated with cytarabine, serum AST and LDH levels rose "transiently and slightly" during the first 48 hours of infusion in 4 patients, but isoenzyme testing suggested that the enzymes were not hepatic in origin).
- Griner PF, Elbadawi A, Packman CH. Veno-occlusive disease of the liver after chemotherapy of acute leukemia. Report of two cases. Ann Intern Med 1976; 85: 578-82. [PubMed: 1068643](Two patients with acute leukemia developed fatal sinusoidal obstruction syndrome after combination cyclic chemotherapy; the only two agents that both had received were thioguanine and cytarabine, thioguanine being the more likely culprit).
- Cassileth PA, Katz ME. Chemotherapy for adult acute nonlymphocytic leukemia with daunorubicin and cytosine arabinoside. Cancer Treat Rep 1977; 61: 1441-5. [PubMed: 922749](Among 21 adults with acute leukemia treated with cytarabine and daunorubicin, one-third had bilirubin elevations after the initial course, but serum enzymes were normal and the elevations did not recur with a second course).
- Herzig RH, Wolff SN, Lazarus HM, Phillips GL, Karanes C, Herzig GP. High-dose cytosine arabinoside therapy for refractory leukemia. Blood 1983; 62: 361-9. [PubMed: 6223674](Among 57 patients with refractory leukemia treated with high doses of cytarabine [3 g/m2 every 12 hours], elevations in liver tests occurred in almost all patients and were moderate in severity in 75%, but severe only in those with longer courses of therapy).
- Pizzuto J, Avilés A, Ramos E, Cervera J, Aguirre J. Cytosine arabinoside induced liver damage: histopathologic demonstration. Med Pediatr Oncol 1983; 11: 287-90. [PubMed: 6577264](A 50 year old woman and 33 year old man with AML were treated with cycles of cytarabine [dose not given], vincristine and prednisone and developed liver test elevations after the second course [peak direct bilirubin ~10 and 5 mg/dL, AST ~1000 and 475 U/L], with greater elevations after a third course, resolving rapidly after stopping, and biopsies showing mild nonspecific inflammation and focal necrosis).
- George CB, Mansour RP, Redmond J 3rd, Gandara DR. Hepatic dysfunction and jaundice following high-dose cytosine arabinoside. Cancer 1984; 54: 2360-2. [PubMed: 6594185](Two men, ages 45 and 31, with refractory leukemia were treated with high doses of cytarabine [3 g/m2 every 12 hours for 6 days] and developed jaundice within 2 weeks [bilirubin 21 and 23 mg/dL, AST 29 and 10 U/L, Alk P 393 and 103 U/L], both dying of septic shock before recovery; autopsy showed passive congestion and minimal changes).
- Donehower RC, Karp JE, Burke PJ. Pharmacology and toxicity of high-dose cytarabine by 72-hour continuous infusion. Cancer Treat Rep 1986; 70: 1059-65. [PubMed: 3461882](28 patients with refractory leukemia received varying doses of cytarabine [4 to 24 g/m2 over 72 hours], enzymes elevations occurred in 89% and values greater than 5 times ULN in 7%; "The toxicity of this schedule...was formidable at the higher doses").
- Kantarjian HM, Estey EH, Plunkett W, Keating MJ, Walters RS, Iacoboni S, McCredie KB, Freireich EJ. Phase I-II clinical and pharmacologic studies of high-dose cytosine arabinoside in refractory leukemia. Am J Med 1986; 81: 387-94. [PubMed: 3463209](Among 64 patients with refractory leukemia treated with high dose cytarabine [3 g/m2 every 12 hours for 4-12 doses], liver tests abnormalities occurred in 14 [2%] and were severe in 3 [2%]).
- Sznol M, Ohnuma T, Holland JF. Hepatic toxicity of drugs used for hematologic neoplasia. Semin Liver Dis 1987; 7: 237-56. [PubMed: 3317861](Review of the hepatotoxicity of drugs used to treat leukemia mentions that low doses of cytarabine cause mild serum enzyme elevations in 5-10% of patients, but that higher doses can cause abnormalities with jaundice in a high proportion of patients).
- Bolwell BJ, Cassileth PA, Gale RP. Low dose cytosine arabinoside in myelodysplasia and acute myelogenous leukemia: a review. Leukemia 1987; 1: 575-9. [PubMed: 3312846](Systematic review of "low dose" cytarabine therapy of AML and myelodysplasia, identified 463 patients in 10 studies; toxicity was largely due to myelosuppression, and "there were no reports of cerebellar or hepatic toxicity" which were common with high dose therapy).
- Bolwell BJ, Cassileth PA, Gale RP. High dose cytarabine: a review. Leukemia 1988; 2: 253-60. [PubMed: 3287015](Systematic review of results of trials of high dose cytarabine therapy in acute leukemia, toxicities include prolonged pancytopenia, cerebellar dysfunction, peripheral neuropathy and gastrointestinal problems including abnormal liver tests in 9-72% of patients, which are usually mild and reversible, but two deaths associated with cholestatic jaundice have been reported [George 1984]).
- Tanaka H, Kawakami M, Kuraishi Y, Meguro S, Ishikawa E. A comparative pathological study of liver injury after different combination chemotherapies for leukemia. Acta Pathol Jpn 1988; 38: 1417-32. [PubMed: 3223277](Use of a regimen of daunorubicin, cytarabine, mercaptopurine and prednisone was associated with a distinctive hepatic injury marked by irreversible liver cell injury and hemosiderosis that was no longer seen once thioguanine was used instead of mercaptopurine).
- Hashimoto M, Seekikawa I, Saito K, Horie S, Ishiyama T, Sugimoto M, Wakabayashi Y, Hirose S. [Acute leukemia complicated by hyperbilirubinemia due to high dose cytosine arabinoside therapy]. Rinsho Ketsueki 1989; 30: 262-5. Japanese. [PubMed: 2746881](Abstract: 58 year old man with acute leukemia developed jaundice with normal ALT levels after a course of cytarabine, autopsy showing cholestasis without necrosis).
- Kirtley DW, Votaw ML, Thomas E. Jaundice and hepatorenal syndrome associated with cytosine arabinoside. J Natl Med Assoc 1990; 82: 209, 213, 217-8. [PMC free article: PMC2626026] [PubMed: 2319616](28 year old man with acute leukemia in relapse was treated with cytarabine [3 g/m2 every 12 hours for 9 doses], developing fever and jaundice [bilirubin 9.2 rising to 45 mg/dL, ALT 88 U/L, Alk P 55 U/L], followed by progressive multiorgan failure and dying within 11 days of starting therapy; autopsy showed bile stasis and centrolobular steatosis).
- Nachbaur K, Dietze O, Herold M, Thaler J, Braunsteiner H, Vogel W. Fulminant hepatic failure after high-dose cytosine arabinoside and mitoxantrone treatment for relapse of acute myelogenous leukaemia. Eur J Haematol 1992; 49: 221-3. [PubMed: 1464366](15 year old girl with relapse of AML given high dose cytarabine and mitoxantrone developed progressive rise in ALT 14 days after chemotherapy [bilirubin 2.2 mg/dL, ALT 110 rising to 3330 U/L, Alk P 324 U/L, LDH 15030 U/L], with hepatic coma and death, autopsy showing massive centrolobular necrosis).
- Wysocki M, Nowaczyk-Michalak A, Pilecki O, Trybu. L, Balcar-Boro. A. Jaundice following high-dose arabinoside cytosine in a child with acute myelogenous leukemia. Acta Haematol Pol 1992; 23: 197-9. [PubMed: 1492544](Child with AML developed jaundice after 3rd and 4th course of cytarabine, which resolved on stopping therapy).
- Faggioli P, De Paschale M, Tocci A, Luoni M, Fava S, De Paoli A, Tosi A, et al. Acute hepatic toxicity during cyclic chemotherapy in non Hodgkin's lymphoma. Haematologica 1997; 82: 38-42. [PubMed: 9107080](Among 98 Italian patients with lymphoma treated with cyclic chemotherapy [7 receiving cytarabine with other agents], 12 developed acute hepatitis including 8 of 22 who were HBsAg positive, 1 of 11 anti-HCV positive, but only 3 of 76 negative for both markers).
- Tanaka M, Kanamori H, Yamaji S, Mishima A, Fujita H, Fujisawa S, Murata T, et al. Low-dose cytarabine-induced hepatic and renal dysfunction in a patient with myelodysplastic syndrome. Anticancer Drugs 1999; 10: 289-91. [PubMed: 10327034](48 year old woman with myelodysplasia developed fever after 12 days during an initial course of subcutaneous cytarabine [every 12 hours], with rapid recurrence on restarting followed by renal and hepatic injury [bilirubin 1.3 mg/dL, ALT 232 U/L, creatinine 4.4], which resolved upon stopping and reoccurred again rapidly with an attempt at a third course).
- Altundag O, Altundag K, Celik I, Turker A, Kars A. Isolated hyperbilirubinemia following standard dose cytosine arabinoside in a patient with relapsed acute myeloid leukemia. Am J Hematol 2004; 75: 263-4. [PubMed: 15054828](56 year old woman with relapsed AML developed sepsis and jaundice 11 days after starting cytarabine and idarubicin [bilirubin 12.5 rising to 42.9 mg/dL, ALT 0 U/L, Alk P 145 U/L], resolving with resolution of neutropenia).
- Babaoglu MO, Karadag O, Saikawa Y, Altundag K, Elkiran T, Yasar U, Bozkurt A. Hepatotoxicity due to a possible interaction between cytosine arabinoside and dipyridamole: a case report. Eur J Clin Pharmacol 2004; 60: 455-6. [PubMed: 15232664](52 year old man with relapse of acute leukemia was treated with high dose cytarabine while receiving dipyridamole, and developed fever and jaundice and died of septic shock; the authors hypothesize interaction between the two drugs resulting in higher cytarabine levels).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis 2006; 38: 33-8. [PubMed: 16054882](Survey of drug induced liver fatalities reported to WHO database between 1968-2003 revealed 4690 reports [89% from the US]; cytarabine was associated with 53 cases).
- Coutsouvelis J, Corallo CE. The management of prolonged, isolated hyperbilirubinemia following cytarabine-based chemotherapy for acute myeloid leukaemia. J Oncol Pharm Pract 2009; 15: 107-10. [PubMed: 18818219](47 year old man with AML developed jaundice with normal ALT and Alk P levels after a course of cytarabine, idarubicin and etoposide [bilirubin 1.6 on day 8 rising to 14.9 mg/dL on day 26], course complicated by sepsis and hypotension).
- Advani AS, McDonough S, Coutre S, Wood B, Radich J, Mims M, O'Donnell M, et al. SWOG S0910: a phase 2 trial of clofarabine/cytarabine/epratuzumab for elapsed/refractory acute lymphocytic leukaemia. Br J Haematol 2014; 165: 504-9. [PMC free article: PMC4209396] [PubMed: 24579885](Among 35 adults with refractory ALL treated with clofarabine, cytarabine and epratuzumab [anti-CD22], the response rate was 52%, but adverse events were common, including ALT elevations above 5 times ULN in 11 patients [31%], one of whom had "hepatic failure", but there were no liver related deaths).
- Martínez-Cuadrón D, Montesinos P, Oriol A, Salamero O, Vidriales B, Bergua J, Herrera P, et al. Phase II trial to assess the safety and efficacy of clofarabine in combination with low-dose cytarabine in elderly patients with acute myeloid leukemia. Ann Hematol 2014; 93: 43-6. [PubMed: 24081577](Among 11 elderly adults with AML treated with clofarabine with low doses of cytarabine, the response rate was 27%, but the mortality rate rose to 73% at 8 weeks and one death was attributed to hepatic and renal failure).
- Donadieu J, Bernard F, van Noesel M, Barkaoui M, Bardet O, Mura R, Arico M, et al; Salvage Group of the Histiocyte Society. Cladribine and cytarabine in refractory multisystem Langerhans cell histiocytosis: results of an international phase 2 study. Blood 2015; 126: 1415-23. [PMC free article: PMC4624454] [PubMed: 26194764](Among 27 patients with refractory Langerhans cell histiocytosis treated with two 5-day courses of cytarabine and cladribine, the major toxicities were pancytopenia and sepsis; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. (Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 [5.5%] [PMC free article: PMC4446235] [PubMed: 25754159]were attributed to antineoplastic agents, but none were due to cytarabine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Azacitidine.[LiverTox: Clinical and Researc...]Review Azacitidine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Fludarabine.[LiverTox: Clinical and Researc...]Review Fludarabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Clofarabine.[LiverTox: Clinical and Researc...]Review Clofarabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Decitabine.[LiverTox: Clinical and Researc...]Review Decitabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Daunorubicin.[LiverTox: Clinical and Researc...]Review Daunorubicin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Cytarabine - LiverToxCytarabine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...