NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ivacaftor, lumacaftor, tezacaftor and elexacaftor are orally available potentiators or correctors of the cystic fibrosis transmembrane conductance regulator (CFTR) that are used to treat patients with cystic fibrosis with specific mutations of the CFTR. Ivacaftor alone or in combination with lumacaftor or tezacaftor has been associated with low rate of transient serum enzyme elevations during treatment, but not with clinically apparent acute liver injury with jaundice, while the addition of elexacaftor to ivacaftor and tezacaftor has been implicated in a higher rate of serum enzyme elevations (up to 10% of patients) and to rare instances of clinically apparent liver injury with jaundice.
Background
Cystic fibrosis (CF) is a severe inherited disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator gene (CFTR), which results in impaired clearance of mucous secretions leading to progressive pancreatic and pulmonary dysfunction, considerable disability and early mortality. CF is considered the most common, fatal genetic disorder among Caucasians, affecting approximately 1:2000 persons of European descent. Disease manifestations generally arise in childhood and include pancreatic insufficiency, poor nutrition, failure to thrive and progressive lung disease with frequent respiratory infections and pulmonary exacerbations. Survival is poor, but has improved greatly with medical interventions and attention to maintenance of rigorous pulmonary hygiene, preventive or rapid treatment of respiratory infections and proper nutritional management. The discovery that the disease was caused by mutations in the CFTR gene led to a focused search for small molecules that might improve, correct or potentiate abnormal CFTR function. A major problem was the diversity of mutations found in CF and the variability in how these mutations affected gene function (proper folding of the mature CFTR protein, trafficking of the protein to the proper place in the plasma membrane, channel opening and maintenance of the open configuration). Nevertheless, several agents were identified that were potentiators or correctors ("tor") of the CFTR ("caf") and could improve respiratory function, sense of well being and nutrition and decrease pulmonary exacerbations in patients with CF who had agent-specific mutations in the CFTR gene. Currently, four “caftors” are available clinically – ivacaftor, lumacaftor, tezacaftor and elexacaftor. The combination of three of these modulators – elexacaftor, tezacaftor and ivacaftor – has become the standard of care and is effective in improving pulmonary function and symptoms in up to 85% of patients, a major milestone in management of this important genetic disease.
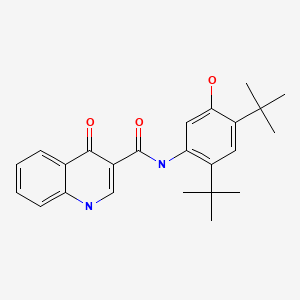
Ivacaftor (eye" va kaf' tor) was the first CFTR modulator to become available for use in the United States. It potentiates the opening of the CFTR channel in patients who harbor at least one mutation in the CFTR gene that is responsive to ivacaftor based upon clinical or in vitro assay data, such as the Gly551Asp (also abbreviated as G551D) CFTR mutation. Ivacaftor was approved in the United States for use in patients with the CFTR Gly551Asp mutation in 2012 and is available as monotherapy in tablets of 150 mg and as oral granules in packets of 50 and 75 mg under the brand name Kalydeco. The recommended dose in adults and children above 6 years of age is 150 mg orally every 12 hours. The dose in children less than 6 years of age is based upon body weight. The Gly551Asp mutation is found in approximately 5% of patients with CF.
Lumacaftor (loo" ma kaf' tor) is a "corrector" of the CFTR and was the second agent to gain approval as therapy of CF, but was approved for use only in combination with ivacaftor, and specifically for patients who are homozygous for the Phe508del (F508del) mutation in the CFTR. Phen508del is the most frequent mutation in CFTR found in patients with CF and is associated with a lack of trafficking of the transporter to the cell surface. In vitro, lumacaftor was found to partially correct this trafficking error. Lumacaftor combined with ivacaftor was approved for use in patients homozygous for Phe508del CFTR in 2015 and is available as tablets consisting of 200 mg of lumacaftor and 125 mg of ivacaftor under the brand name Orkambi. Side effects of these agents are generally mild, but can include headache, nasal congestion, abdominal pain, diarrhea, nausea, dizziness and rash.
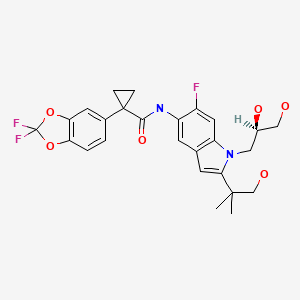
Tezacaftor (tez" a kaf' tor) is a "corrector" of the CFTR and was the third agent to gain approval, but only in combination with ivacaftor, and specifically for patients who are homozygous for the Phe508Del (F508del) mutation in the CFTR or are heterozygous and have another mutation in CFTR found in patients with CF and is associated with a lack of trafficking of the transporter to the cell surface. In vitro, tezacaftor was found to partially correct this trafficking error. Tezacaftor combined with ivacaftor was approved for use in the United States as therapy for patients with cystic fibrosis in 2018 and is available as tablets of fixed dose of 100 mg tezacaftor with 150 mg of ivacaftor co-packaged with tablets of 150 mg of ivacaftor alone under the brand name Symdeko. Side effects of these agents are generally mild but can include headache, nasal congestion, abdominal pain, diarrhea, nausea, dizziness and rash.
Elexacaftor (el ex” a kaf’ tor) is also a “corrector” of the CFTR and was the fourth agent to gain approval, but only in combination with ivacaftor and tezacaftor (triple therapy). Initially evaluated in patients who were homozygous for the Phe508del allele, further trials showed that it was effective in patients with only one Phe508 allele and a variety of second mutated alleles. Clinical trials of this triple combination therapy was found to rapidly improve pulmonary function tests and clinical symptoms resulting in a decrease in acute exacerbations and sweat chloride concentrations, weight gain and improved pulmonary function and quality of life. Triple therapy with elexacaftor, tezacaftor and ivacaftor was approved for use in patients with cystic fibrosis aged 12 years or older with at least one Phen508del allele in the United States in 2019 under the brand name Trikafta. The recommended dosage is two tablets of a fixed combination of 100 mg of elexacaftor, 50 mg of tezacaftor, and 75 mg of ivacaftor each morning and a second tablet of ivacaftor 150 mg alone each evening. Because approximately 85% of patients with cystic fibrosis have at least one Phe508del allele, triple therapy is applicable to most patients and has replaced previous combinations of “caftors” as the standard of therapy.
Common side effects of triple therapy include an initial worsening of cough, rhinorrhea, sinusitis, nasal congestion and dyspnea, elevations in ALT, bilirubin and CPK, abdominal pain, diarrhea, and rash. Less common but potentially severe complications include cataracts, pancreatitis and acute cholecystitis.
Hepatotoxicity
In large randomized controlled trials of ivacaftor with or without lumacaftor or tezacaftor, up to 25% of subjects had some degree of serum aminotransferase elevations during therapy. The elevations, however, were generally transient and mild and were above 3 times the upper limit of normal (ULN) in only 2% to 5% of patients. The abnormalities were usually asymptomatic and often resolved spontaneously without dose adjustment. Furthermore, in several studies, similar rates of serum enzyme elevations were noted in the placebo treated groups. Nevertheless, serum aminotransferase elevations resulted in dose modification or interruption in 1% to 2% of patients on ivacaftor alone or with lumacaftor or tezacaftor.
In prelicensure clinical trials of the triple therapy of elexacaftor, tezacaftor and ivacaftor, higher rates of serum enzyme elevations were reported which in some cases were accompanied by elevations in serum bilirubin. Most abnormalities resolved spontaneously and enzymes rapidly improved upon discontinuation. Since approval of triple therapy, several case reports of clinically apparent liver injury including jaundice have been reported, generally arising after weeks or months of treatment and resolving with discontinuation. In addition, case series of acute cholecystitis developing within days or weeks of starting triple therapy have been reported, and are probably due to the increased biliary fluid secretion and clearance of biliary sludge and stones with the sudden increased bile flow. Gallstones are frequent in patients with cystic fibrosis and often result in cholecystectomy. There have been no reports of acute liver failure, chronic hepatitis or vanishing bile duct syndrome linked to elexacaftor, tezacaftor and ivacaftor therapy. Patients with cystic fibrosis can, not infrequently, have underlying liver disease with chronic, intermittent serum aminotransferase and alkaline phosphatase elevations, occasionally associated with progressive fibrosis or nodular regenerative hyperplasia and non-cirrhotic portal hypertension.
Combination of elexacaftor, tezacaftor, and ivacaftor likelihood score: D (possible cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which elexacaftor with tezacaftor and ivacaftor might cause liver injury is not known. Ivacaftor, tezacaftor and elexacaftor are extensively metabolized by the liver by the cytochrome P450 system (largely CYP 3A) and liver injury might be caused by a toxic or immunogenic product of their metabolism. Triple therapy is susceptible to drug-drug interactions, particularly with strong inducers (such as rifampin and St. John's wort), which may result in suboptimal drug levels or strong inhibitors of CYP 3A4 (such as ketoconazole) that might result in higher than normal drug levels.
Outcome and Management
While chronic therapy with elexacaftor, tezacaftor and ivacaftor can be associated with mild-to-moderate serum aminotransferase elevations, they have rarely been linked to cases of clinically apparent liver injury. Monitoring of routine liver tests is recommended for patients starting triple therapy with repeat testing while on therapy every 3 months for one year and annually thereafter. Patients who develop aminotransferase elevations on therapy should be monitored more frequently, and dosing should be interrupted if symptoms or jaundice appear or if aminotransferase levels rise above 5 times ULN, with caution in restarting once they fall into the normal range. The safety and efficacy of these agents in patients with CF and significant liver disease has not been demonstrated and increased monitoring and caution is appropriate in patients with preexisting liver abnormalities.
Drug Class: Genetic Disorder Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ivacaftor – Kalydeco®
Lumacaftor/Ivacaftor – Orkambi®
Tezacaftor/Ivacaftor – Symdeko®
Elexacaftor/Tezacaftor/ Ivacaftor – Trikafta®
DRUG CLASS
Cystic Fibrosis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ivacaftor | 873054-44-5 | C24-H28-N2-O3 |
|
| Lumacaftor | 936727-05-8 | C24-H18-F2-N2-O5 |

|
| Tezacaftor | 1152311-62-0 | C26-H27-F3-N2-O6 |
|
| Elexacaftor | 2216712-66-0 | C26-H34-F3-N7-O4-S |

|
ANNOTATED BIBLIOGRAPHY
References updated: 26 August 2021
Abbreviations used: CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator.
- Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevínek P, Griese M, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–72. [PMC free article: PMC3230303] [PubMed: 22047557](Among 161 patients with CF and the Gly551Asp mutation of CFTR, pulmonary function improved more and respiratory exacerbations were less in those who were treated with ivacaftor compared to placebo; serum ALT levels above 2 times ULN occurred in 9.6% of ivacaftor vs 11.6% of placebo recipients, and were above 5 times ULN in 3.6% vs 1.3%).
- Davis PB. Therapy for cystic fibrosis--the end of the beginning? N Engl J Med. 2011;365:1734–5. [PubMed: 22047565](Editorial in response to Ramsey [2011]).
- Hebestreit H, Sauer-Heilborn A, Fischer R, Käng M, Mainz JG. Effects of ivacaftor on severely ill patients with cystic fibrosis carrying a G551D mutation. J Cyst Fibros. 2013;12:599–603. [PubMed: 23757359](Among 14 patients with severe lung disease due to CF who were treated with ivacaftor for up to 1 year, 1 developed ALT elevations of 3 to 4 times ULN, 1 had AST elevations and 1 bilirubin elevations, but all resolved spontaneously without drug discontinuation).
- Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, Mainz JG, et al. VX08-770-103 (ENVISION) Study Group. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187:1219–25. [PMC free article: PMC3734608] [PubMed: 23590265](Among 52 children with CF and CFTR mutation Gly551Asp treated with ivacaftor or placebo for 48 weeks, FEV1 improved significantly in ivacaftor, but not placebo recipients [12.6% vs 0.1%], while adverse event rates were similar and there were "no clinically important trends attributable to ivacaftor" in liver test results).
- Wood ME, Smith DJ, Reid DW, Masel PJ, France MW, Bell SC. Ivacaftor in severe cystic fibrosis lung disease and a G551D mutation. Respirol Case Rep. 2013;1:52–4. [PMC free article: PMC4184528] [PubMed: 25473543](Among 3 adults with CF, CFTR Gly551Asp mutations and severe lung disease who were treated with ivacaftor for 24 weeks, one developed "elevated liver enzymes" which required drug withdrawal, but later tolerated restarting ivacaftor without recurrence of liver injury).
- Holmes D. False dawn for cystic fibrosis disease modifiers? Nat Rev Drug Discov. 2014;13:713–4. [PubMed: 25270946](Editorial on the results of trials of ivacaftor/lumacaftor combination therapy focusing on the modest effects achieved [2.6-4% increase in FEV1] and recent in vitro data suggesting that ivacaftor may reverse the effects of lumacaftor on the Phe508del mutant CFTR).
- McKone EF, Borowitz D, Drevinek P, Griese M, Konstan MW, Wainwright C, Ratjen F, et al. VX08-770-105 (PERSIST) Study Group. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST). Lancet Respir Med. 2014;2:902–10. [PubMed: 25311995](Among 192 adults and children with CF and Gly551Asp mutant CFTR who were treated in an extension study after the 48 week-controlled trials of ivacaftor [Ramsey 2011, Davies 2013], efficacy was maintained and there were no new safety concerns, although 9 patients [5%] developed ALT elevations above 5 times ULN [without bilirubin elevations] and required dose interruptions).
- Boyle MP, Bell SC, Konstan MW, McColley SA, Rowe SM, Rietschel E, Huang X, et al. VX09-809-102 study group. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med. 2014;2:527–38. [PubMed: 24973281](In a dose finding study of lumacaftor alone and in combination with ivacaftor vs placebo in 188 patients with CF and the Phe508del mutant CFTR, there was little change in lung function [FEV1] and one patient developed ALT elevations, but before starting active therapy).
- Hayes D Jr, Warren PS, McCoy KS, Sheikh SI. Improvement of hepatic steatosis in cystic fibrosis with ivacaftor therapy. J Pediatr Gastroenterol Nutr. 2015;60:578–9. [PubMed: 25688481](17 year old girl with Gly551Asp mutant CFTR and hepatic steatosis was treated with ivacaftor for 2 years and was found to have resolution of the steatosis by MR imaging, but had also received ursodiol which was associated temporarily with improvements in serum enzyme elevations).
- Taylor-Cousar J, Niknian M, Gilmartin G, Pilewski JM. for the VX11-770-901 investigators. Effect of ivacaftor in patients with advanced cystic fibrosis and a G551D-CFTR mutation: Safety and efficacy in an expanded access program in the United States. J Cyst Fibros. 2016;15(1):116–22. [PubMed: 25682022](Among 44 adults and children with CF [Gly551Asp mutant CFTR] and severe lung disease who were treated with ivacaftor for 24 weeks, FEV1 improved by an average of 5.5% and most patients gained weight; liver test abnormalities were noted in 1 patient [2%], but no details were provided).
- Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, et al. TRAFFIC and TRANSPORT Study Groups. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–31. [PMC free article: PMC4764353] [PubMed: 25981758](Among 1108 adult and adolescent patients with CF who were homozygous for the Phe508del CFTR mutant allele and were treated with the combination of lumacaftor and ivacaftor for 24 weeks, ALT elevations above 3 times ULN occurred in 5.2% of patients on the drug combination vs 5.1% on placebo; drug discontinuations for liver test elevations occurred in 7 patients on the drug combination vs none on placebo, and concurrent ALT and bilirubin elevations occurred only in drug-treated patients [n=3]).
- Jones AM, Barry PJ. Lumacaftor/ivacaftor for patients homozygous for Phe508del-CFTR: should we curb our enthusiasm? Thorax. 2015;70:615–6. [PubMed: 26071414](Commentary on the safety and efficacy of the combination of lumacaftor with ivacaftor in patients with CF [with the Phe508del mutation in the CFTR] mentions the rather modest improvements in lung function found in the controlled trials of the combination [Wainwright 2015], and the finding of significant elevations in both ALT and bilirubin levels in 3 treated patients raising the issue of interaction between the two agents).
- Moss RB, Flume PA, Elborn JS, Cooke J, Rowe SM, McColley SA, Rubenstein RC, et al. VX11-770-110 (KONDUCT) Study Group. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir Med. 2015;3:524–33. [PMC free article: PMC4641035] [PubMed: 26070913](Among 69 adults and children with CF and the Arg117His mutant CFTR treated with ivacaftor or placebo for 24 weeks, sweat chloride levels decreased with treatment, but FEV1 results did not improve significantly; no mention of ALT elevations or clinically apparent liver injury).
- Elborn JS, Ramsey BW, Boyle MP, Konstan MW, Huang X, Marigowda G, Waltz D, et al. VX-809 TRAFFIC and TRANSPORT study groups. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis. Lancet Respir Med. 2016;4:617–26. [PMC free article: PMC6612264] [PubMed: 27298017](Pooled analysis of two placebo controlled trials of lumacaftor/ivacaftor in 1108 patients with CF [and Phe508del CFTR mutation], focuses on improvements in pulmonary function test results; no mention of ALT elevations, hepatotoxicity or non-pulmonary serious adverse events).
- Hubert D, Dehillotte C, Munck A, David V, Baek J, Mely L, Dominique S, et al. Retrospective observational study of French patients with cystic fibrosis and a Gly551Asp-CFTR mutation after 1 and 2 years of treatment with ivacaftor in a real-world setting. J Cyst Fibros. 2018;17(1):89–95. [PubMed: 28711222](Among 57 patients with CF [and Gly551Asp-CFTR mutation] treated with ivacaftor for 1-2 years, "no significant adverse events were reported", although therapy was discontinued in 2 patients because of abnormal liver enzyme levels or cirrhosis).
- Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, Milla CE, et al. VX14-809-109 investigator group. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6-11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2017;5:557–67. [PubMed: 28606620](Among 204 children with CF [homozygous for Phen508del CFTR mutation] treated with lumacaftor/ ivacaftor or placebo, pulmonary function tests improved more frequently in drug treated children, while overall adverse event rates were similar; ALT or AST elevations above 3 times ULN occurred in 13% vs 8%, and above 5 times in 5% vs 3%, although all liver enzyme elevations were self-limited and not accompanied by jaundice or symptoms).
- Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, Huang X, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med. 2017;5:107–18. [PubMed: 28011037](Among 340 patients with CF [Phen508del CFTR mutation] who continued lumacaftor/ivacaftor therapy after participation in randomized controlled trials [Wainwright 2015; Elborn 2016], the beneficial effects of therapy appeared to be maintained while ALT or AST elevations above 3 times ULN arose in 8% of patients, but all elevations were self-limited and none were accompanied by bilirubin elevations; one subject developed acute hepatitis E).
- Rowe SM, McColley SA, Rietschel E, Li X, Bell SC, Konstan MW, Marigowda G, Waltz D, Boyle MP. VX09-809-102 Study Group. Lumacaftor/ivacaftor treatment of patients with cystic fibrosis heterozygous for F508del-CFTR. Ann Am Thorac Soc. 2017;14:213–9. [PMC free article: PMC5461999] [PubMed: 27898234](Among 126 patients with CF [heterozygous for phe508del CFTR mutation] treated with lumacaftor/ivacaftor for 56 days, overall side effect rates were similar in the two groups; one drug treated subject discontinued therapy because of ALT and AST elevations).
- Milla CE, Ratjen F, Marigowda G, Liu F, Waltz D, Rosenfeld M. VX13-809-011 Part B Investigator Group *. Lumacaftor/ivacaftor in patients aged 6-11 years with cystic fibrosis and homozygous for F508del-CFTR. Am J Respir Crit Care Med. 2017;195:912–20. [PMC free article: PMC5440888] [PubMed: 27805836](Among 56 children with CF [homozygous for phe508del] treated with lumacaftor/ivacaftor for 24 weeks, ALT or AST elevations above 5 times ULN occurred in 5 patients [9%], and 3 stopped therapy because of enzyme elevations, but none developed jaundice or symptoms).
- Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, Nair N, et al. Tezacaftor-Ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med. 2017;377:2024–35. [PMC free article: PMC6472479] [PubMed: 29099333](Among 248 patients with cystic fibrosis heterozygous for the Phe508del mutation in CFTR treated in a cross over study of tezacaftor/ivacaftor vs ivacaftor alone vs placebo, improvements in lung function were greater with active treatment, but adverse event rates were similar in all 3 groups, ALT elevations above 3 times ULN occurring in 11.7% of tezacaftor/ivacaftor, 13.4% on ivacaftor alone and 8% on placebo, but none were accompanied by jaundice or led to early discontinuation).
- Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, Wang LT, et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for phe508del. N Engl J Med. 2017;377:2013–23. [PubMed: 29099344](Among 509 patients with cystic fibrosis who were homozygous for phe508del who were treated with tezacaftor/ivacaftor or placebo for 24 weeks improvement in lung function was treated with the active drug than with placebo, while adverse event rates were similar, ALT elevations above 3 times ULN occurring in 4.7% vs 3.2% and leading to early discontinuation in 0.4% vs 0.2% of subjects).
- Tezacaftor/Ivacaftor (Symdeko) for cystic fibrosis. Med Lett Drugs Ther. 2018;60(1558):174–6. [PubMed: 30335045](Concise summary of the mechanism of action, clinical efficacy, safety and costs of tezacaftor/ivacaftor; mentions that serum aminotransferase levels can occur and that monitoring of liver tests should be done before starting therapy followed by every 3 months for the first year and annually thereafter).
- Davies JC, Moskowitz SM, Brown C, Horsley A, Mall MA, McKone EF, Plant BJ, et al. VX16-659-101 Study Group. VX-659-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1599–611. [PMC free article: PMC6277022] [PubMed: 30334693](Among 117 selected patients with cystic fibrosis treated with tezacaftor/ivacaftor with or without VX-659 or placebo, improvements in lung function and sweat chloride were greatest with triple therapy, but that 3 of 49 patients receiving triple therapy developed ALT elevations above 3 times ULN, but the abnormalities resolved even without dose reduction).
- Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, et al. VX16-445-001 Study Group.VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1612–20. [PMC free article: PMC6289290] [PubMed: 30334692](Among 123 selected patients with cystic fibrosis treated with tezacaftor/ivacaftor with or without VX445 or placebo, improvements in lung function and sweat chloride were greatest with triple therapy and adverse event rates were similar in all groups, ALT or AST elevations occurring in 28% receiving VX445, 28% receiving tezacaftor/ivacaftor and 33% on placebo and were above 5 times ULN in only 1 subject).
- Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, Ramsey BW, et al. VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381:1809–1819. [PMC free article: PMC7282384] [PubMed: 31697873](Among 402 patients with cystic fibrosis [age 12 years or older] with one Phe508del allele and one minimal function allele treated with triple therapy [elexacaftor, tezacaftor, ivacaftor] vs placebo for 24 weeks, pulmonary function tests and symptoms improved and acute exacerbations and sweat chloride concentrations decreased while adverse events were generally mild and only slightly greater than placebo, ALT elevations arising in 11% vs 4% and being above 3 times ULN in 8% vs 5.5%, 5 times ULN in 2.5% vs 1.5% and 8 times ULN in 1.5% vs 1% with no early discontinuations because of liver test abnormalities and no accompanying jaundice).
- Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, Mall MA, et al. VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–1948. [PMC free article: PMC7571408] [PubMed: 31679946](Among 107 patients with cystic fibrosis and homozygous for the F508del mutation were treated with tezacaftor and ivacaftor with or without elexacaftor for 4 weeks, pulmonary function tests and sweat chloride improved with addition of elexacaftor and adverse event rates were similar, although ALT elevations above 3 times ULN occurred in 4 elexacaftor treated [7%] versus none given placebo; there were no severe hepatic adverse events or cases of clinically apparent liver injury).
- Hoy SM. Elexacaftor/Ivacaftor/Tezacaftor: first approval. Drugs. 2019;79:2001–2007. [PubMed: 31784874](Review of the mechanism of action, history of development, clinical efficacy and safety of triple therapy using ivacaftor, tezacaftor, and elexacaftor shortly after the approval of this regimen as therapy of cystic fibrosis in the US, mentions that ALT elevations occurred in a small proportion of treated patients at a higher rate than with placebo).
- Elexacaftor/tezacaftor/ivacaftor (Trikafta) for cystic fibrosis. Med Lett Drugs Ther. 2020;62(1589):5–7. [PubMed: 31999662](Concise review of the mechanisms of action, clinical efficacy, safety and costs of triple therapy of elexacaftor, tezacaftor and ivacaftor for cystic fibrosis shortly after its approval in the US mentions that adverse events leading to discontinuation occurred in 1% of patients and aminotransferase elevations above twice the upper limit of normal arose in 11% [vs 4% with placebo] and bilirubin elevations in 4% [vs <1%]).
- Safirstein J, Grant JJ, Clausen E, Savant D, Dezube R, Hong G. Biliary disease and cholecystectomy after initiation of elexacaftor/ivacaftor/tezacaftor in adults with cystic fibrosis. J Cyst Fibros. 2021;20:506–510. [PubMed: 32736949](Case series of 7 patients with cystic fibrosis who developed biliary colic within 1-27 days after starting the combination of elexacaftor, ivacaftor, and tezacaftor, 6 of whom underwent cholecystectomy and most were then able to tolerate triple therapy without further biliary pain).
- Brennan S, Marmor I, Schafer C, Ko J, Torres Garcia JA, Rosman IS, Coughlin C, et al. Serum sickness-like reaction following initiation of elexacaftor/tezacaftor/ivacaftor therapy. Pediatr Pulmonol. 2020;55:2846–2847. [PubMed: 32956574](12 year old boy with cystic fibrosis developed nausea, abdominal pain, fever, pruritus and rash starting 5 days after initiation of elexacaftor, tezacaftor and ivacaftor triple therapy with fever, joint swelling and leukocytosis without eosinophilia, creatinine or ALT elevations, responding to a 3 week course of prednisone).
- Hu MK, Wood G, Dempsey O. 'Triple therapy' (elexacaftor, tezacaftor, ivacaftor) skin rash in patients with cystic fibrosis. Postgrad Med J. 2020 Dec 11: postgradmedj-2020-139264. Epub ahead of print. [PubMed: 33310895](24 year old woman with cystic fibrosis developed generalized rash 8 days after starting triple therapy with elexacaftor, tezacaftor and ivacaftor, which resolved rapidly with discontinuation and corticosteroid therapy and did not recur when she was restarted with a slow initial titration).
- Griese M, Costa S, Linnemann RW, Mall MA, McKone EF, Polineni D, Quon BS, et al. Safety and efficacy of elexacaftor/tezacaftor/ivacaftor for 24 weeks or longer in people with cystic fibrosis and one or more F508del alleles: interim results of an open-label phase 3 clinical trial. Am J Respir Crit Care Med. 2021;203:381–385. [PMC free article: PMC8020728] [PubMed: 32969708](Among 506 patients with cystic fibrosis enrolled in an ongoing open-label extension study of triple therapy [elexacaftor, tezacaftor, ivacaftor], frequent adverse reactions included paradoxical exacerbation of pulmonary disease, cough and sore throat and rash in 5%, early discontinuations in 7 patients 4 for ALT elevations and 1 for rash, while ALT elevations occurred in 36 [7%] of patients, that were above 3 times ULN in 6%, 5 times ULN in 2%, and 8 times ULN in 0.6%).
- Stylemans D, François S, Vincken S, Verbanck S, Vanderhelst E. A case of self-limited drug induced liver injury under treatment with elexacaftor/tezacaftor/ivacaftor: When it is worth taking the risk. J Cyst Fibros. 2021;20:712–714. [PubMed: 34134936](58 year old woman with severe cystic fibrosis developed enzyme elevations 1-2 weeks after starting triple therapy [elexacaftor, tezacaftor and ivacaftor] that rose to ALT of 15 times ULN and Alk P of 5 times ULN but normal bilirubin; stopping therapy resulted in improvement and restarting in worsening, but eventually with initial low dose treatment, full dose therapy was tolerated with normal liver tests).
- Zemanick ET, Taylor-Cousar JL, Davies J, Gibson RL, Mall MA, McKone EF, McNally P, et al. A phase 3 open-label study of elexacaftor/tezacaftor/ivacaftor in children 6 through 11 years of age with cystic fibrosis and at least one F508del allele. Am J Respir Crit Care Med. 2021;203:1522–1532. [PMC free article: PMC8483230] [PubMed: 33734030](Among 66 children [ages 6 to 11] with cystic fibrosis and at least one F508del allele treated with triple therapy [elexacaftor, tezacaftor, and ivacaftor] for 24 weeks, pulmonary function and clinical symptoms improved and adverse events, while common [98%], were usually transient and mild-to-moderate in severity; ALT or AST elevations above times ULN arose in 7 children but only one was above 5 times ULN and none required dose interruption; rash occurred in 24% but resolved without stopping therapy in all but one).
- Lowry S, Mogayzel PJ, Oshima K, Karnsakul W. Drug-induced liver injury from elexacaftor/ivacaftor/ tezacaftor. J Cyst Fibros. 2021:S1569-1993(21)01297-2. Epub ahead of print. [PubMed: 34275759](21 year old woman with cystic fibrosis was found to have abnormal liver tests 5 months after starting triple therapy [elexacaftor, tezacaftor, ivacaftor], which worsened despite stopping treatment with peak ALT 2707 U/L and bilirubin 3.1 mg/dL one month later, biopsy showing moderate inflammation and centrolobular necrosis but with eventual resolution and normal liver tests 6 months later).
- Barry PJ, Mall MA, Álvarez A, Colombo C, de Winter-de Groot KM, Fajac I, McBennett KA, et al. VX18-445-104 Study Group. Triple therapy for cystic fibrosis Phe508del-gating and -residual function genotypes. N Engl J Med. 2021;385:815–825. [PMC free article: PMC8982185] [PubMed: 34437784](Among 132 patients [ages 12 years or more] with cystic fibrosis and one Phe508del allele and a second allele with either a gating- or a residual function genotype treated with triple therapy [elexacaftor, tezacaftor, ivacaftor] or an active control therapy [ivacaftor alone or with tezacaftor], pulmonary function and symptoms improved more with triple therapy while adverse events were generally similar, ALT elevations arising in 6% vs none and resulting in early discontinuation in one patient; rash arose in 3% vs 4%).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Correctors (specific therapies for class II CFTR mutations) for cystic fibrosis.[Cochrane Database Syst Rev. 2018]Review Correctors (specific therapies for class II CFTR mutations) for cystic fibrosis.Southern KW, Patel S, Sinha IP, Nevitt SJ. Cochrane Database Syst Rev. 2018 Aug 2; 8(8):CD010966. Epub 2018 Aug 2.
- Physiologically Based Pharmacokinetic Modeling of CFTR Modulation in People with Cystic Fibrosis Transitioning from Mono or Dual Regimens to Triple-Combination Elexacaftor/Tezacaftor/Ivacaftor.[Pulm Ther. 2020]Physiologically Based Pharmacokinetic Modeling of CFTR Modulation in People with Cystic Fibrosis Transitioning from Mono or Dual Regimens to Triple-Combination Elexacaftor/Tezacaftor/Ivacaftor.Tsai A, Wu SP, Haseltine E, Kumar S, Moskowitz SM, Panorchan P, Shah K. Pulm Ther. 2020 Dec; 6(2):275-286. Epub 2020 Jul 30.
- The rescue of F508del-CFTR by elexacaftor/tezacaftor/ivacaftor (Trikafta) in human airway epithelial cells is underestimated due to the presence of ivacaftor.[Eur Respir J. 2022]The rescue of F508del-CFTR by elexacaftor/tezacaftor/ivacaftor (Trikafta) in human airway epithelial cells is underestimated due to the presence of ivacaftor.Becq F, Mirval S, Carrez T, Lévêque M, Billet A, Coraux C, Sage E, Cantereau A. Eur Respir J. 2022 Feb; 59(2). Epub 2022 Feb 24.
- Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: a 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial.[Lancet Respir Med. 2022]Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: a 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial.Sutharsan S, McKone EF, Downey DG, Duckers J, MacGregor G, Tullis E, Van Braeckel E, Wainwright CE, Watson D, Ahluwalia N, et al. Lancet Respir Med. 2022 Mar; 10(3):267-277. Epub 2021 Dec 20.
- Review The preclinical discovery and development of the combination of ivacaftor + tezacaftor used to treat cystic fibrosis.[Expert Opin Drug Discov. 2020]Review The preclinical discovery and development of the combination of ivacaftor + tezacaftor used to treat cystic fibrosis.Guerra L, Favia M, Di Gioia S, Laselva O, Bisogno A, Casavola V, Colombo C, Conese M. Expert Opin Drug Discov. 2020 Aug; 15(8):873-891. Epub 2020 Apr 15.
- Cystic Fibrosis Agents - LiverToxCystic Fibrosis Agents - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...