NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Cysteamine is a simple aminothiol molecule that is used to treat nephropathic cystinosis, due to its ability to decrease the markedly elevated and toxic levels of intracellular cystine that occur in this disease and cause its major complications. Cysteamine has been associated with serum enzyme elevations when given intravenously in high doses, but it has not been shown to cause clinically apparent acute liver injury.
Background
Cysteamine (sis tee' a meen) is a simple aminothiol molecule which is used to treat nephropathic cystinosis, a rare autosomal recessive disorder characterized by progressive renal tubular dysfunction and growth retardation, and which eventually leads to renal failure and need for dialysis or renal transplantation often within the first decade of life. The underlying defect in cystinosis is a deficiency of an amino acid carrier that transports cystine out of lysosomes. The absence of this transporter causes accumulation of toxic levels of cystine in tissues. Those organs most susceptible to the toxic effects of cystine include the kidney, endocrine tissue, eyes and muscle. Cysteamine readily penetrates cells and is concentrated in lysosomes where it acts by converting intralysosomal cystine to cysteine which is readily transported out of lysosomes. This action leads to a decrease in cellular cystine levels and prevents the renal failure, growth retardation and many of the other chronic complications of nephropathic cystinosis. Cysteamine was approved for the therapy of cystinosis in the United States in 1994. It is now available in several forms including cysteamine bitartrate salt under the commercial name Cystagon (given every 6 hours), as well as a delayed release preparation under the name ProCysBi (given every 12 hours). The typical maintenance dose is 2.0 grams daily in adults and children above the age of 12, and 1.3 to 1.95 grams/m2 daily in younger children. Cysteamine is usually initiated using a fraction of the maintenance dose, with gradual dose escalation. Both preparations of cysteamine have an unpleasant taste and odor which makes compliance with taking the high doses required to control intracellular cystine levels in cystinosis a frequent problem. Common side effects include nausea, anorexia, fatigue and diarrhea. Rare, but potentially severe adverse reactions include rash, skin fragility and benign intracranial hypertension. Joint hyperextension, leg pains, osteopenia, fractures and scoliosis also occur in cysteamine treated patients with cystinosis, but are more likely complications of the underlying disease than adverse effects due to cysteamine.
Hepatotoxicity
During long term use of cysteamine in preregistration studies, serum ALT elevations occurred in a small proportion of treated subjects, but the background rate of serum enzyme elevations in this population is high and was not defined in the open label studies. There have been reports of more marked enzyme elevations during high dose therapy with cysteamine, with recurrence on reexposure. These abnormalities were invariably asymptomatic and rapidly reversed with dose adjustment. In addition, patients with cystinosis appear to be at risk for developing nodular regenerative hyperplasia and noncirrhotic portal hypertension. The role of long term cysteamine therapy in these hepatic complications is not clear. There have been no reports of clinically apparent, acute liver injury with jaundice attributable to cysteamine, although it has had only limited wide scale use.
Likelihood score: D[HD] (possible cause of liver injury when given in high doses).
Mechanism of Injury
The mechanism by which cysteamine might lead to serum enzyme elevations or liver injury is not known. Cysteamine is metabolized in most cells and it does not seem to be a substrate for or affect the hepatic cytochrome P450 system. In clinical trials of cysteamine, no evidence of drug-drug interactions was identified.
Outcome and Management
Serum enzyme elevations during cysteamine therapy are generally mild and self-limited, resolving even without drug interruption or dose reduction. Other causes of liver injury should be sought before assuming that the abnormalities are due to cysteamine.
Drug Class: Genetic Disorder Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cysteamine Bitartrate – Cystagon®
Cysteamine Bitartrate (Delayed Release) – ProCysBi®
DRUG CLASS
Genetic Disorder Agents
Product labeling at DailyMed, National Library of Medicine, NIH
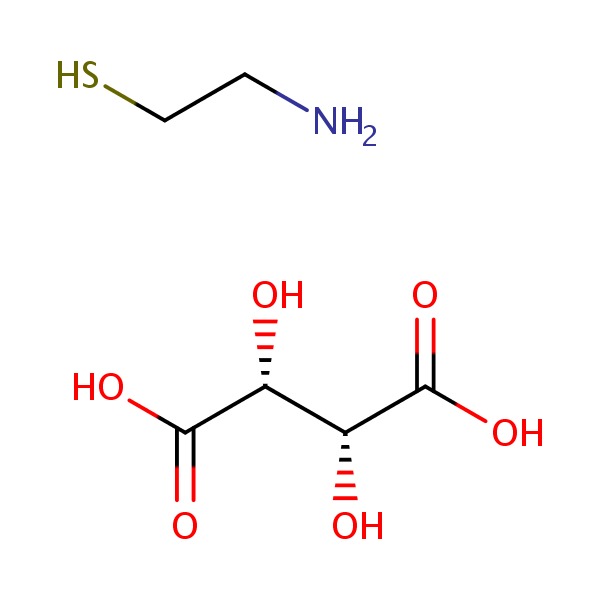
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Cysteamine | 27761-19-9 | C4-H6-O6.C2-H7-N-S |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 03 November 2017
- Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 731-4.(Expert review of hepatotoxicity published in 1999; cysteamine is not discussed).
- Gahl WA, Bashan N, Tietze F, Bernardini I, Schulman JD. Cystine transport is defective in isolated leukocyte lysosomes from patients with cystinosis. Science 1982; 217 (4566): 1263-5. [PubMed: 7112129](Demonstration of defective transporter for cystine in lysosomes from patients with cystinosis which results in a toxic accumulation of cystine; in contrast, the egress of methionine, tryptophan and other amino acids is normal, suggesting a specific amino acid transporter defect).
- Avner ED, Ellis D, Jaffee R. Veno-occlusive disease of the liver associated with cysteamine treatment of nephropathic cystinosis. J Pediatr 1983; 102: 793-6. [PubMed: 6842343](8 year old boy with nephropathic cystinosis developed ascites and gastrointestinal bleeding from gastric varices 22 months after starting cysteamine [bilirubin not given, ALT 16 U/L, Alk P 68 U/L, protime 19 sec], biopsy showing changes of sinusoidal obstruction syndrome).
- Gahl WA, Schulman JD, Thoene JG, Schneider J. Hepatotoxicity of cysteamine? J Pediatr 1983; 103: 1008-9. [PubMed: 6644413](Letter in response to Avner [1983] suggesting that sinusoidal obstruction syndrome may be a part of the clinical spectrum of cystinosis, rather than a complication of cysteamine therapy).
- Gahl WA, Schneider JA, Thoene JG, Chesney R. Course of nephropathic cystinosis after age 10 years. J Pediatr 1986; 109: 605-8. [PubMed: 3531450](Among 80 patients with nephropathic cystinosis above the age of 10 identified in a US wide survey, 90% had undergone renal transplant [at an average age of 10 years] and the oldest child without transplant was 14 and on dialysis; other complications included hepatomegaly [42%] and splenomegaly, but liver tests were abnormal in only 11 [14%] who had mild ALT or AST elevations; other late complications included photophobia, decreased visual acuity and hypothyroidism).
- Gahl WA, Reed GF, Thoene JG, Schulman JD, Rizzo WB, Jonas AJ, Denman DW, et al. Cysteamine therapy for children with nephropathic cystinosis. N Engl J Med 1987; 316: 971-7. [PubMed: 3550461](Among 93 children with nephropathic cystinosis treated with cysteamine hydrochloride [mean dose 51 mg/kg daily] for up to 6 years, leucocyte cystine concentrations decreased by 82%, renal function stabilized and growth was improved in comparison to historical controls who were not treated; side effects included nausea [from its taste and odor] and one child developed sinusoidal obstruction syndrome which was attributed to the underlying disease, rather than cysteamine).
- Gahl WA, Thoene JG, Schneider JA, O'Regan S, Kaiser-Kupfer MI, Kuwabara T. NIH conference. Cystinosis: progress in a prototypic disease. Ann Intern Med 1988; 109: 557-69. [PubMed: 3048161](Review of the clinical characteristics, epidemiology, pathogenesis, complications and therapy of cystinosis).
- Kimonis VE, Troendle JR, Rose SR, Yang ML, Markello TC, Gahl WA. Effects of early cysteamine therapy on thyroid function and growth in nephropathic cystinosis. J Clin Endocrinol Metab 1995; 80: 3257-61. [PubMed: 7593434](Among 101 patients with nephropathic cystinosis treated with cysteamine, those started on therapy before the age of 2 and who maintained optimal leukocyte cystine levels [n=24] had lower rates of hypothyroidism and better growth and bone age than those with partial [n=36] or poor or delayed [n=47] treatment responses).
- Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med 2002; 347: 111-21. [PubMed: 12110740](Review of the history, clinical characteristics, clinical variants, complications, genetics, diagnosis and treatment of cystinosis).
- Kleta R, Gahl WA. Pharmacological treatment of nephropathic cystinosis with cysteamine. Expert Opin Pharmacother 2004; 5: 2255-62. [PubMed: 15500372](Review of cysteamine therapy of cystinosis; states that early diagnosis and therapy with cysteamine can guarantee normal growth and maintenance of renal function).
- Kleta R, Bernardini I, Ueda M, Varade WS, Phornphutkul C, Krasnewich D, Gahl WA. Long-term follow-up of well-treated nephropathic cystinosis patients. J Pediatr 2004; 145: 555-60. [PubMed: 15480385](Long term course of two siblings with cystinosis who were started on cysteamine therapy at 2 and 20 months of age, and both achieved normal growth and maintained normal renal function [creatinine clearance of 78 and 105 mL/min] for 8 and 12 years).
- Kleta R, Kaskel F, Dohil R, Goodyer P, Guay-Woodford LM, Harms E, Ingelfinger JR, et al.; NIH Office of Rare Diseases. First NIH/Office of Rare Diseases Conference on Cystinosis: past, present, and future. Pediatr Nephrol 2005; 20: 452-4. [PubMed: 15747161](Brief summary of the major clinical challenges in cystinosis including early diagnosis, improved therapeutics, better compliance and transition of care from pediatric to adult medical services).
- O'Brien K, Hussain N, Warady BA, Kleiner DE, Kleta R, Bernardini I, Heller T, et al. Nodular regenerative hyperplasia and severe portal hypertension in cystinosis. Clin Gastroenterol Hepatol 2006; 4: 387-94. [PubMed: 16527704](Two adults, ages 19 and 30 years, with nephropathic cystinosis but minimal exposure to cysteamine were found to have severe noncirrhotic portal hypertension, and liver biopsies demonstrated nodular regenerative hyperplasia with minimal portal fibrosis, the etiology of which was unknown).
- Gahl WA, Balog JZ, Kleta R. Nephropathic cystinosis in adults: natural history and effects of oral cysteamine therapy. Ann Intern Med 2007; 147: 242-50. [PubMed: 17709758](Among 100 adults with nephropathic cystinosis, 92 had a kidney transplants and at least half had hypothyroidism, hypergonadism, pulmonary insufficiency, swallowing disorders and myopathy, one-third had retinopathy and one quarter diabetes; cysteamine therapy was associated with delay in need for renal allograft and fewer complications and deaths).
- Bendel-Stenzel MR, Steinke J, Dohil R, Kim Y. Intravenous delivery of cysteamine for the treatment of cystinosis: association with hepatotoxicity. Pediatr Nephrol 2008; 23: 311-5. [PubMed: 17668247](Two year old girl with severe nephropathic cystinosis and intolerance to oral cysteamine was treated with an intravenous form [5-6 mg/kg every 8 hours] which resulted in decreases in intracellular cystine, but was followed after 50 days by marked elevations in serum ALT [2117 U/L] and AST [1362 U/L] without jaundice [bilirubin 0.4 mg/dL], levels promptly falling on stopping the infusions and recurring with intravenous rechallenge [ALT ~140 U/L after 25 days], and later with oral rechallenge using cysteamine bitartrate [ALT 249 U/L after 6 months]).
- Besouw MT, Bowker R, Dutertre JP, Emma F, Gahl WA, Greco M, Lilien MR, et al. Cysteamine toxicity in patients with cystinosis. J Pediatr 2011; 159: 1004-11. [PubMed: 21784456](Among 550 European patients with cystinosis who were treated with cysteamine, 8 developed possible toxicity, 6 of whom developed vascular lesions over the elbow marked by irregular collagen fibers and angioendotheliomatosis, other complications were leg pains and neurologic symptoms which improved with lowering the dose; possibly due to inhibition of cross linking of collagen).
- Dohil R, Schmeltzer S, Cabrera BL, Wang T, Durelle J, Duke KB, Schwimmer JB, et al. Enteric-coated cysteamine for the treatment of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2011; 33: 1036-44. [PubMed: 21395631](Among 11 children [>10 years] with nonalcoholic fatty liver disease and raised serum ALT levels treated with enteric coated cysteamine [15 mg/kg daily], mean ALT levels fell [from 120 to 55 U/L], with no change in weight and rise of ALT values after stopping therapy [to 74 U/L).
- Langman CB, Greenbaum LA, Sarwal M, Grimm P, Niaudet P, Deschênes G, Cornelissen E, et al. A randomized controlled crossover trial with delayed-release cysteamine bitartrate in nephropathic cystinosis: effectiveness on white blood cell cystine levels and comparison of safety. Clin J Am Soc Nephrol 2012; 7: 1112-20. [PMC free article: PMC3386675] [PubMed: 22554716](Among 43 patients with cystinosis treated with standard cysteamine [every 6 hours] or a new formulation of enteric coated, delayed release form of cysteamine bitartrate [every 12 hours] for 3 weeks with subsequent cross over, mean peak white cell cystine levels were similar, but gastrointestinal side effects were greater with the delayed release form).
- Schwimmer JB, Lavine JE, Wilson LA, Neuschwander-Tetri BA, Xanthakos SA, Kohil R, Barlow S, et al. In children with nonalcoholic fatty liver disease, cysteamine bitartrate delayed-release improves liver enzymes but does not reduce disease activity scores. Gastroenterol 2016: 151: 1141-54. [PMC free article: PMC5124386] [PubMed: 27569726](Among 169 children with nonalcoholic fatty liver treated with cysteamine [150 to 450 mg daily] or placebo for 48 weeks, ALT and AST levels improved significantly, but histologic changes were minimal; no patient developed clinically worsening or clinically apparent liver injury).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- A comparison of immediate release and delayed release cysteamine in 17 patients with nephropathic cystinosis.[Orphanet J Rare Dis. 2021]A comparison of immediate release and delayed release cysteamine in 17 patients with nephropathic cystinosis.van Stein C, Klank S, Grüneberg M, Ottolenghi C, Grebe J, Reunert J, Harms E, Marquardt T. Orphanet J Rare Dis. 2021 Sep 14; 16(1):387. Epub 2021 Sep 14.
- A comparison of the effectiveness of cysteamine and phosphocysteamine in elevating plasma cysteamine concentration and decreasing leukocyte free cystine in nephropathic cystinosis.[Pediatr Res. 1988]A comparison of the effectiveness of cysteamine and phosphocysteamine in elevating plasma cysteamine concentration and decreasing leukocyte free cystine in nephropathic cystinosis.Smolin LA, Clark KF, Thoene JG, Gahl WA, Schneider JA. Pediatr Res. 1988 Jun; 23(6):616-20.
- Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo.[J Clin Invest. 1976]Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo.Thoene JG, Oshima RG, Crawhall JC, Olson DL, Schneider JA. J Clin Invest. 1976 Jul; 58(1):180-9.
- Review Pharmacological treatment of nephropathic cystinosis with cysteamine.[Expert Opin Pharmacother. 2004]Review Pharmacological treatment of nephropathic cystinosis with cysteamine.Kleta R, Gahl WA. Expert Opin Pharmacother. 2004 Nov; 5(11):2255-62.
- Review Early oral cysteamine therapy for nephropathic cystinosis.[Eur J Pediatr. 2003]Review Early oral cysteamine therapy for nephropathic cystinosis.Gahl WA. Eur J Pediatr. 2003 Dec; 162 Suppl 1:S38-41. Epub 2003 Nov 11.
- Cysteamine - LiverToxCysteamine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...