NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Fluorouracil (5-FU) is a pyrimidine analogue used as an antineoplastic agent to treat multiple solid tumors including colon, rectal, breast, gastric, pancreatic, ovarian, bladder and liver cancer. Fluorouracil is associated with a low rate of transient serum aminotransferase elevations during therapy and has been implicated in rare cases of clinically apparent acute liver injury.
Background
Fluorouracil (floo" oh ure' a sil) is a fluoropyrimidine that has antineoplastic action against several solid tumors including breast and colon cancers. Fluorouracil is believed to block thymidylate synthase and decrease production of thymidylate, a necessary precursor of DNA. This action interferes with the synthesis of DNA, RNA and protein and blocks cell division. Fluorouracil was approved for use as an anticancer agent in the United States in 1962 and is currently used as an important component of several anticancer drug regimens. Fluorouracil is typically combined with leucovorin (folinic acid) which also inhibits thymidylate synthase, thus enhancing the effects of fluorouracil. Current indications for fluorouracil with leucovorin include palliative therapy of advanced breast, bladder, colon, rectum, liver, pancreas and stomach cancer. Fluorouracil is available in various sized vials of 50 mg/mL generically and under several brand names including Adrucil and Carac. The typical recommended dose is 12 mg/kg given intravenously daily for 4 days and, if tolerated, again on days 6, 8 and 12 as a part of 30 day cycles of chemotherapy. In addition, fluorouracil has been used as a continuous hepatic arterial infusion in management of hepatic metastases from colorectal and other cancers. Fluorouracil is also available topically in creams and solutions for therapy of actinic keratoses and basal cell cancers. Common side effects of intravenous therapy include bone marrow suppression, fatigue, weakness, headache, dizziness, insomnia, paresthesias, abdominal pain, constipation, diarrhea, dyspepsia, nausea, stomatitis, rash, and hand-foot syndrome.
Hepatotoxicity
Several forms of hepatotoxicity have been associated with fluorouracil therapy. Serum aminotransferase elevations occur in up to 70% of patients treated with cyclic courses of fluorouracil, the rate of abnormalities being partially dose related. The aminotransferase elevations are usually transient and mild, are not commonly above 5 times the upper limit of normal and rarely associated with symptoms. The elevations may be associated with hepatic steatosis, which can be demonstrated by hepatic imaging and has been confirmed by liver biopsies that show variable degrees of macrovesicular steatosis and portal inflammation. The injury rarely leads to clinically apparent liver injury. Isolated case reports of clinically apparent acute liver injury with jaundice attributed to fluorouracil have been published, but such cases are rare and the relatedness to fluorouracil remains unclear. Intrahepatic artery infusion of fluorouracil is also associated with serum aminotransferase elevations or chemical hepatitis (in 5% to 7% of cases) and in biliary abnormalities, but it rarely causes biliary strictures or the sclerosing cholangitis-like syndrome that is common with floxuridine (FUDR) treatment. In systematic reviews of the literature, biliary strictures suggestive of sclerosing cholangitis have occurred in <1% of patients treated with hepatic arterial infusions of fluorouracil as compared to 5% to 12% of those given floxuridine. Infusions of fluorouracil can also result in marked shrinkage of metastatic tumors in the liver and partial atrophy of the noninvolved liver, resulting in an irregular lobular appearance suggestive of cirrhosis.
A separate and distinct syndrome associated with fluorouracil therapy is the rapid development of coma with hyperammonemia, lactic acid elevations and respiratory alkalosis that occurs most frequently with continuous infusions of high doses of fluorouracil. The onset is generally within 1 to 2 days of starting infusions and is marked by progressive confusion and stupor followed by coma. Standard liver tests such as ALT, AST, alkaline phosphatase and bilirubin are usually normal, but plasma ammonia levels are high (generally >250 μg/dL) and accompanied by varying degrees of respiratory alkalosis, elevations in serum lactate and azotemia. Infectious complications are common, but most patients recover rapidly with hydration and supportive care. Patients who develop this syndrome can later tolerate lower doses of fluorouracil, but recurrences have been reported. The mortality rate is approximately 10%, most deaths being due to sepsis rather than hepatic failure. The cause of the syndrome is not known, but it is associated with higher doses of chemotherapy. This syndrome has not been reliably linked to other pyrimidine analogues such as capecitabine.
Likelihood score: A (well known cause of clinically apparent liver injury).
Mechanism of Injury
While severe hepatotoxicity from fluorouracil may be rare, when it occurs it is likely due to direct or intrinsic hepatotoxicity. The injury may be caused by inhibition of thymidylate synthase. In addition, fluorouracil is extensively metabolized in the liver via the microsomal enzyme system, and production of a toxic intermediate may trigger liver injury. The hyperammonemic coma that occurs with high dose infusions of fluorouracil is likely due to inhibition of mitochondrial function, which may be particularly clinically manifest in persons with an underlying, inherited but subclinical mitochondrial enzyme defect. However, fluorouracil readily crosses the blood-brain barrier and some of the toxicity may be due to a direct effect on the central nervous system.
Outcome and Management
The severity of the liver injury linked to fluorouracil therapy is generally mild. Fluorouracil has not been linked to cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome. There is no information on cross sensitivity to hepatic injury between fluorouracil and other pyrimidine analogues.
Drug Class: Antineoplastic Agents
Other Drugs in the Subclass, Pyrimidine Analogues: Azacitidine, Capecitabine, Cytarabine, Decitabine, Floxuridine, Gemcitabine, Trifluridine/Tipracil
CASE REPORT
Case 1. Hyperammonemic coma due to fluorouracil.
[Modified from: Advani PP, Fakih MG. 5-FU-induced hyperammonemic encephalopathy in a case of metastatic rectal adenocarcinoid successfully rechallenged with the fluoropyrimidine analog, capecitabine. Anticancer Res 2011; 31: 335-8. PubMed Citation]
A 70 year old woman with metastatic colorectal cancer developed confusion and coma 2 days after a second course of fluorouracil, leucovorin and bevacizumab. She had been treated in the previous year with combination chemotherapy, including fluorouracil (2 g/m2), folinic acid, oxaliplatin and bevacizumab as 46 hour continuous infusions every 2 weeks. Because of gastrointestinal toxicity, the chemotherapy was discontinued, but repeat imaging suggested disease progression and she was restarted on fluorouracil, folinic acid and bevacizumab. Two days after finishing the second cycle of this modified regimen, she developed nausea, vomiting, weakness, diarrhea and confusion. Her mental status deteriorated and she developed coma. She had no known history of liver or neurologic disease. Her other medications included atenolol, hydrochlorothiazide, omeprazole, dexamethasone, warfarin, aprepitant and multivitamins. Laboratory tests showed normal complete blood counts and electrolytes. The serum bilirubin was 0.6 mg/dL, ALT 50 U/L, AST 54 U/L and alkaline phosphatase 79 U/L (all within the normal range). Serum ammonia, however, was markedly elevated at 203 μmol/L (normal 11-51). Computerized tomography of the head was normal. She was treated with supportive care and serum ammonia levels fell into the normal range within 24 hours. She improved clinically and was discharged. In follow up, fluorouracil and leucovorin were stopped and she was treated with capecitabine and bevacizumab. She had no recurrence of symptoms and plasma ammonia levels remained normal on multiple occasions.
Key Points
| Medication: | Fluorouracil (2.4 g/m2 daily as 46 hour infusions) |
|---|---|
| Pattern: | None |
| Severity: | Coma |
| Latency: | 3-4 days |
| Recovery: | Complete within 24 to 48 hours |
| Other medications: | Leucovorin, bevacizumab, atenolol, hydrochlorothiazide, omeprazole, warfarin, multivitamins |
Comment
An elderly woman with advanced, metastatic colorectal cancer developed hyperammonemic coma within a few days of starting a second cycle of fluorouracil with leucovorin and bevacizumab. Although she had metastatic liver disease, all liver tests were normal. Lactic acid levels and arterial blood gases were not reported. As in other cases of hyperammonemic coma due to fluorouracil, however, recovery was rapid even with simple supportive therapy including hydration and monitoring. In many instances, there is recurrence if fluorouracil is restarted. This report was important in that capecitabine (a prodrug of fluorouracil) therapy was not associated with recurrence. The cause of the hyperammonemia is unknown, but it is likely from the liver rather than brain, and is probably due to direct inhibition of mitochondrial function and urea cycle enzyme activity by the pyrimidine analogue. Dehydration may play an accessory role in this syndrome.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Fluorouracil – Generic, Adrucil®, Carac®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Fluorouracil | 51-21-8 | C4-H3-F-N2-O2 |
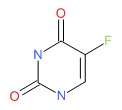 |
ANNOTATED BIBLIOGRAPHY
References updated: 02 February 2018
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; mentions that fluorouracil can cause liver injury which is typically hepatocellular and likely to be intrinsic [direct]).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3r ed. Amsterdam: Elsevier, 2013, p. 541-67.(Review of hepatotoxicity of cancer chemotherapeutic agents).
- Chabner BA, Bertino J, Cleary J, Ortiz T, Lane A, Supko JG, Ryan DPl. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1677-730.(Textbook of pharmacology and therapeutics).
- Bateman JR, Pugh RP, Cassidy FR, Marshall GJ, Irwin LE. 5-fluorouracil given once weekly: comparison of intravenous and oral administration. Cancer 1971; 28: 907-13. [PubMed: 5111743](Comparison of once weekly intravenous vs oral 5-FU therapy in 107 patients with advanced solid organ tumors; hepatic toxicity was "seen infrequently").
- Massey WH, Fletcher WS, Judkins MP, Dennis DL. Hepatic artery infusion for metastatic malignancy using percutaneously placed catheters. Am J Surg 1971; 121: 160-4. [PubMed: 4100063](Description of use of percutaneous placed hepatic artery catheters for administration of 5-FU to patients with metastatic cancer; two patients developed hepatic coma who had "pre-coma" before therapy).
- Smith JP, Randall GE, Castro JR, Lindberg RD. Hypogastric artery infusion and radiation therapy for advanced squamous cell carcinoma of the cervix. Am J Roentgenol Radium Ther Nucl Med 1972; 114: 110-5. [PubMed: 5009403](Pilot study of use of surgically implanted gastric artery catheter for administration of 5-FU with leucovorin or methotrexate to 63 patients with metastatic or advanced cancer, reported 3 patients with mild, 6 with moderate and 4 with severe liver toxicity).
- Vestfrid MA, Castelleto L, Giméz PO. [Diffuse liver necrosis in treatment with 5-fluorouracil]. Rev Clin Esp 1972; 125: 549-50. [PubMed: 5074970](29 year old man with a mesothelioma developed nausea within 4 days of starting intravenous fluorouracil [1 mg daily] and died two days later, autopsy showing massive centrolobular necrosis).
- Vaughan WP, Wilcox PM, Alderson PO, Ettinger DS, Abeloff MD. Hepatic toxicity of adjuvant chemotherapy for carcinoma of the breast. Med Pediatr Oncol 1979; 7: 351-9. [PubMed: 547161](Among 24 women with advanced breast cancer who received chemotherapy with 5-FU, cyclophosphamide and methotrexate, 4 developed hepatotoxicity as marked by mild Alk P elevations and abnormal liver scans 3-20 months after starting therapy, suggestive of tumor but showing inflammation only by liver biopsy, resolving upon stopping chemotherapy).
- Daly JM, Kemeny N, Oderman P, Botet J. Long-term hepatic arterial infusion chemotherapy. Arch Surg 1984; 119: 935-41. [PubMed: 6378147](40 patients with metastatic colorectal cancer underwent hepatic arterial infusions with FUDR; toxic effects were largely gastrointestinal with ulcers [30%], gastritis without ulcers [18%], elevations in bilirubin >3 mg/dL [23%], AST [65%], and extrahepatic bile duct strictures [5%]).
- Kemeny MM, Goldberg DA, Browning S, Metter GE, Miner PJ, Terz JJ. Experience with continuous regional chemotherapy and hepatic resection as treatment of hepatic metastases from colorectal primaries. A prospective randomized study. Cancer 1985; 55: 1265-70. [PubMed: 3971296](Among 46 patients with hepatic metastases from colorectal cancer treated with continuous hepatic artery infusions of FUDR, 58% had chemical hepatitis with ALT or AST >3 times ULN and mild jaundice, 8 also developed biliary strictures and 6 of the first 19 required cholecystectomy for cholecystitis; one patient with strictures died of hepatic failure).
- Sugarbaker PH, Gianola FJ, Speyer JC, Wesley R, Barofsky I, Meyers CE. Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery 1985; 98: 414-22. [PubMed: 3898450](Among 65 patients with advance colorectal cancer treated with 12 cycles of increasing doses of 5-FU, ALT elevations occurred in 60% of those given intraperitoneal vs 67% given intravenous 5-FU).
- Caruso U, Elli M, Cravotto E, Tisone G, Lombardo PA, Silvaroli M, Ficorella C, Cortesi E, Casciani CU. Colorectal cancer (Duke's group B2, C1, C2): adjuvant chemotherapy and toxicity. Mt Sinai J Med 1985; 52: 470-2. [PubMed: 3875028](Among 15 patients with colorectal cancer and hepatic metastases treated with cyclic intravenous 5-FU and methyl CCNU, none developed evidence of hepatic toxicity).
- Luquel L, Ekherian JM, De Gramont A, Offenstadt G. [Anuria, hepatocellular insufficiency and bone marrow aplasia after the administration of streptozocin and fluorouracil]. Therapie 1988; 43: 125. French. PubMed Citation. [PubMed: 2970126](52 year old woman with metastatic carcinoid cancer developed anuria and jaundice after a 2nd 5 day course of streptozocin and fluorouracil [bilirubin 11.9 mg/dL, ALT 1280 U/L], dying 8 days later, injury attributed to streptozocin).
- Mitchell RB, Wagner JE, Karp JE, Watson AJ, Brusilow SW, Przepiorka D, Storb R, et al. Syndrome of idiopathic hyperammonemia after high-dose chemotherapy: review of nine cases. Am J Med 1988; 85: 662-7. [PubMed: 3189370](Idiopathic hyperammonemia was found in 5 of 210 patients being treated for leukemia and 3 of 250 patients undergoing bone marrow transplantation; all 9 had disorientation progressing to coma; liver tests were usually normal, ammonia levels were high [mean=589 µmol/L], most were neutropenic, 6 died and 3 survived, chemotherapy included cytarabine, daunomycin, vincristine, amsacrine, etoposide, cyclophosphamide).
- Kemeny N, Seiter K, Martin D, Urmacher C, Niedzwiecki D, Kurtz R, et al. A new syndrome: ascites, hyperbilirubinemia, and hypoalbuminemia after biochemical modulation of fluorouracil with N-phophonacetyl-L-aspartate (PALA). Ann Intern Med 1991; 115: 946-51. [PubMed: 1824554](Among 44 patients with metastatic colon cancer who received fluorouracil with PALA [an inhibitor of pyrimidine synthesis], 5 patients developed ascites usually with mild jaundice and serum enzyme elevations, with hypoalbuminemia and coagulopathy and fatty change on liver biopsy, improving on stopping treatment, suggestive of L-asparaginase hepatotoxicity).
- Stenram U. Hepatotoxicity of fluorouracil with N-phosphonacetyl-L-aspartate. Ann Intern Med 1992; 117: 90-1. [PubMed: 1530700](Letter in response to Kemeny [1991] suggesting that the toxicity was due to 5-FU and its incorporation into hepatic tissue RNA).
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA. Hepatic toxicity associated with fluorouracil plus levamisole adjuvant therapy. J Clin Oncol 1993; 11: 2386-90. [PubMed: 8246027](Among 376 patients with advanced colorectal cancer treated with cycles of 5-FU and levamisole, 149 [40%] developed hepatotoxicity compared to 16% on levamisole alone and 16% of untreated patients, with variable elevations in ALT, Alk P and bilirubin arising 1-12 months after starting chemotherapy and resolving with stopping in all except 7 patients; liver biopsy in 3 patients showed macrovesicular steatosis and imaging suggested fatty liver).
- Klotz HP, Weder W, LargiadèF. Local and systemic toxicity of intra-hepato-arterial chemotherapy for treatment of unresectable liver metastases of colorectal cancer with 5-Fluorouracil and high dose Leucovorin. Helv Chir Acta 1993; 60: 283-6. [PubMed: 8226075](Among 6 patients who received 24 cycles of hepatic artery infusions of fluorouracil, at least one liver test abnormality occurred during 42% of cycles, but all were mild [grade 1] and rapidly resolved).
- Liaw CC, Liaw SJ, Wang CH, Chiu MC, Huang JS. Transient hyperammonemia related to chemotherapy with continuous infusion of high-dose 5-fluorouracil. Anticancer Drugs 1993; 4: 311-5. [PubMed: 8358058](7 patients, 4 men and 3 women, ages 44 to 68, with advanced cancer treated with high dose intravenous 5-FU developed hyperammonemia 1.5-4 days after starting [bilirubin and ALT normal in all, creatinine 0.7-4.6 mg/dL, ammonia 33-2387 µg/dL], resolving rapidly [<2 days]; 1 died of septic shock).
- Yeh KH, Cheng AL. Acute confusion induced by a high-dose infusion of 5-fluorouracil and folinic acid. J Formos Med Assoc 1994; 93: 721-3. [PubMed: 7858459](61 year old man with gastric cancer developed coma 2 days after a second 1 day course of intravenous 5-FU and leucovorin, which lasted 40 hours and recurred after another infusion, ammonia levels not reported).
- Frasci G, Leone F, Monaco M, Cremone L, Sapio U, Faiella F, Espinosa A, et al. 5-Fluorouracil-interferon-alpha 2b adjuvant treatment of Dukes C colorectal cancer. Dis Colon Rectum 1994; 37: 643-50. [PubMed: 8026229](Among 57 patients with advanced colorectal cancer treated with fluorouracil and alpha interferon, 4 [7%] developed transient liver test abnormalities, all of which resolved after therapy was stopped).
- Figueredo AT, Fawcet SE, Molloy DW, Dobranowski J, Paulseth JE. Disabling encephalopathy during 5-fluorouracil and levamisole adjuvant therapy for resected colorectal cancer: a report of two cases. Cancer Invest 1995; 13: 608-11. [PubMed: 7583711](Two of 82 patients treated with 5-FU and levamisole for colorectal cancer developed progressive dementia while receiving chemotherapy that improved minimally upon stopping; liver enzymes were normal and ammonia not tested).
- Langer CJ, Hageboutros A, Kloth DD, Roby D, Shaer AH. Acute encephalopathy attributed to 5-FU. Pharmacotherapy 1996; 16: 311-3. [PubMed: 8820478](65 year old man with adenocarcinoma treated with 5-FU, leucovorin and cisplatin developed lethargy and coma within 48 hours of starting treatment, with normal serum enzymes and no jaundice, which resolved within 1-2 weeks and did not recur with subsequent courses of lower doses of 5-FU).
- Ross P, Norman A, Cunningham D, Webb A, Iveson T, Padhani A, Prendiville J, Watson M, Massey A, Popescu R, Oates J. A prospective randomised trial of protracted venous infusion 5-fluorouracil with or without mitomycin C in advanced colorectal cancer. Ann Oncol 1997; 8: 995-1001. [PubMed: 9402173](Among 200 patients with advanced colorectal cancer treated with 5-FU infusions with or without mitomycin C for up to 24 weeks, common side effects were mucositus, hand-foot syndrome, diarrhea, nausea, alopecia and bone marrow suppression; no mention of ALT elevations or hepatotoxicity).
- Hirvikoski PP, Kumpulainen EJ, Johansson RT. Hepatic toxicity caused by adjuvant CMF/CNF in breast cancer patients and reversal by tamoxifen. Breast Cancer Res Treat 1997; 44: 269-74. [PubMed: 9266107](Among 194 patients with advanced breast cancer who received adjuvant chemotherapy with or without tamoxifen, minor elevations in AST, Alk P and GGT occurred in patients who received cyclophosphamide with methotrexate and 5-FU alone, but not in those who also received tamoxifen, but no patient developed clinically apparent liver injury or stopped therapy because of enzyme elevations alone).
- Yeh KH, Cheng AL. High-dose 5-fluorouracil infusional therapy is associated with hyperammonaemia, lactic acidosis and encephalopathy. Br J Cancer 1997; 75: 464-5. [PMC free article: PMC2063385] [PubMed: 9020500](Among 280 patients with advanced metastatic cancer treated with high doses of infusional 5-FU, 16 [6%] developed encephalopathy 10-30 hours after starting the infusion and lasting 3-72 hours, accompanied by lactic acidosis [4 to >12 mg/dL, normal .1.3] and hyperammonemia [149 to >500 µmol/L, normal .43], all except one patient recovered completely).
- Bygrave HA, Geh JI, Jani Y, Glynne-Jones R. Neurological complications of 5-fluorouracil chemotherapy: case report and review of the literature. Clin Oncol (R Coll Radiol) 1998; 10: 334-6. [PubMed: 9848338](Two patients with adenocarcinoma treated with 5-FU [one also received cis-platinin] developed neurologic syndrome with neuropathy that improved slowly and incompletely after stopping).
- Valik D. Encephalopathy, lactic acidosis, hyperammonaemia and 5-fluorouracil toxicity. Br J Cancer 1998; 77: 1710-2. [PMC free article: PMC2150046] [PubMed: 9635854](Letter in response to Yeh [1997] suggesting that the patients who developed hyperammonemia might have had preexisting, incomplete forms of mitochondrial enzyme defects).
- Locatelli M, Colleoni M, Noberasco C, Nolé, Orlando L, Munzone E, Peruzzotti G, Goldhirsch A. Hepatic toxicity from cyclophosphamide, methotrexate, fluorouracil (CMF regimen). Ann Oncol 1999; 10: 1394-5. [PubMed: 10631475](Among 264 patients treated with adjuvant chemotherapy with cyclophosphamide, methotrexate and 5-FU, 106 [40%] had liver enzyme elevations, but were >5 times ULN in only 20 [8%]; the increases usually occurred with first few courses and were transient in all, returning to normal within 30 days and resulting in dose modification in 11% of cycles, delay in 3% and permanent discontinuation in only 1%).
- Liaw CC, Wang HM, Wang CH, Yang TS, Chen JS, Chang HK, Lin YC, et al. Risk of transient hyperammonemic encephalopathy in cancer patients who received continuous infusion of 5-fluorouracil with the complication of dehydration and infection. Anticancer Drugs 1999; 10: 275-81. [PubMed: 10327032](29 patients with cancer receiving high dose, continuous infusions of 5-FU developed 32 episodes of encephalopathy, arising 0.5-5 days after starting with ammonia 248 to 2387 µg/dL, often accompanied by azotemia or infection, usually resolving within 2 days; ALT, Alk P and bilirubin normal; deaths due to sepsis).
- Soori GS, Oldham RK, Dobbs TW, Bury MJ, Church CK, DePriest C. Chemo-biotherapy with 5-fluorouracil, leucovorin, and alpha interferon in metastatic carcinoma of the colon.a Cancer Biotherapy Research Group [CBRG] phase II study. Cancer Biother Radiopharm 2000; 15: 175-83. [PubMed: 10803323](Among 16 patients with advanced colorectal cancer treated with fluorouracil and leucovorin weekly and alpha interferon thrice weekly, AST elevations occurred in 19% and Alk P and bilirubin in 12%, but it was difficult to separate drug toxicity from tumor progression as a cause of abnormalities).
- Pirzada NA, Ali II, Dafer RM. Fluorouracil-induced neurotoxicity. Ann Pharmacother 2000; 34: 35-8. [PubMed: 10669184](73 year old man with esophageal carcinoma treated with intravenous fluorouracil [1.5 g daily for 4 days] developed confusion 2 days after stopping [ammonia and lactate normal], resolving within 3 days but with residual ataxia).
- Yamano T, Takayasu Y, Nakao N, Kubota A. [Evaluation of hepatic toxicity following high-dose 5-FU arterial infusion chemotherapy: analysis of 42 cases of colorectal liver metastases]. Nihon Igaku Hoshasen Gakkai Zasshi 2000; 60: 94-102. Japanese. [PubMed: 10741116](Among 42 patients with metastatic colorectal cancer treated with hepatic arterial infusions of 5-FU, Alk P levels were elevated in 19 [46%] and CT scans showed fatty liver in 6 patients [14%], in whom biopsies showed fat and portal inflammation; other patients had lobular changes to liver with shrinkage of metastases and atrophic changes in the residual liver).
- Barnett KT, Malafa MP. Complications of hepatic artery infusion: a review of 4580 reported cases. Int J Gastrointest Cancer 2001; 30: 147-60. [PubMed: 12540027](Systematic review of literature on complications of hepatic artery infusion therapy of cancer; in 23 randomized trials, mortality rate 1% with "chemical hepatitis" in 5% using 5-FU alone vs 31% with FUDR alone, biliary toxicity in <1% vs 14% and sclerosing cholangitis in <1% vs 12%).
- Ishii H, Furuse J, Nagase M, Yoshino M, Kawashima M, Satake M, Ogino T, Ikeda H. Hepatic arterial infusion of 5-fluorouracil and extrabeam radiotherapy for liver metastases from pancreatic carcinoma. Hepatogastroenterology 2004; 51: 1175-8. [PubMed: 15239272](Among 17 patients with liver metastases from pancreatic cancer treated with hepatic arterial infusions of 5-FU, none developed "liver dysfunction" from the chemotherapy).
- Takamori H, Kanemitsu K, Tsuji T, Tanaka H, Chikamoto A, Nakahara O, Hiraoka T, et al. 5-fluorouracil intra-arterial infusion combined with systemic gemcitabine for unresectable pancreatic cancer. Pancreas 2005; 30: 223-6. [PubMed: 15782098](24 patients with pancreatic cancer were treated with intraarterial infusions of fluorouracil [to tumor and liver] 5 days a week for 3 weeks of every 4; gastric and duodenal ulcers occurred in 17% and cholangitis in 21%, requiring biliary drainage, but none resulted in death).
- Sandrasegaran K, Alazmi WM, Tann M, Fogel EL, McHenry L, Lehman GA. Chemotherapy-induced sclerosing cholangitis. Clin Radiol 2006; 61: 670-8. [PubMed: 16843750](Description of 11 patients with FUDR [n=9] or 5-FU [n=2] induced sclerosing cholangitis who underwent ERCP at a single institution; onset after 2-132 months of therapy with jaundice, pruritus or liver test abnormalities [bilirubin 0.4 to 17.6 mg/dL, Alk P 191-1106 U/L]).
- Kim YA, Chung HC, Choi HJ, Rha SY, Seong JS, Jeung HC. Intermediate dose 5-fluorouracil-induced encephalopathy. Jpn J Clin Oncol 2006; 36: 55-9. [PubMed: 16436463](68 year old man developed mental changes on the 5th day of a second course of fluorouracil and cisplatin infusions [bilirubin 1.6 mg/dL, ALT 23 U/L, ammonia 354 ug/dL], resolving within 1 day of stopping the infusions recurring in milder form during a 3rd and 4th cycle).
- Heluwaert F, Santre C, Martin C, Hilleret MN, Martin D. [Coma following chemotherapy: is 5FU implicated? Discussion about on case-report]. Gastroenterol Clin Biol 2006; 30: 325-6. French. [PubMed: 16565673](56 year old woman with colon cancer developed confusion followed by coma 5 days after a 24 hour intravenous course of fluorouracil [~2 g] with leucovorin [lactate 13.8 mEq/L, arterial pH 7.35, creatinine 6.4 mg/dL], resolving rapidly with hydration and support, ammonia levels normal when tested at time of recovery).
- Formica V, Leary A, Cunningham D, Chua YJ. 5-Fluorouracil can cross brain-blood barrier and cause encephalopathy: should we expect the same from capecitabine? A case report on capecitabine-induced central neurotoxicity progressing to coma. Cancer Chemother Pharmacol 2006; 58: 276-8. [PubMed: 16333678](46 year old woman with colon cancer developed disorientation and coma during the 4th cycle of capecitabine and oxaliplatin adjuvant therapy, but with normal liver tests and ammonia, resolving with stopping capecitabine).
- Alazmi WM, McHenry L, Watkins JL, Fogel EL, Schmidt S, Sherman S, Lehman GL. Chemotherapy-induced sclerosing cholangitis: long-term response to endoscopic therapy. J Clin Gastroenterol. 2006; 40: 353-7. [PubMed: 16633109](Description of 11 patients with FUDR [n=9] or 5-FU [n=2] induced sclerosing cholangitis who underwent ERCP at a single institution, strictures were typically in the common hepatic duct near the bifurcation).
- Nott L, Price TJ, Pittman K, Patterson K, Fletcher J. Hyperammonemia encephalopathy: an important cause of neurological deterioration following chemotherapy. Leuk Lymphoma 2007; 48: 1702-11. [PubMed: 17786705](Review of hyperammonemic coma which can occur with cancer chemotherapy [usually fluorouracil] or after bone marrow transplant).
- Brooks AJ, Begg EJ, Chapman BA, Fitzharris BM. Two cases of severe liver injury possibly related to 5-fluorouracil and calcium folinate. Intern Med J 2007; 37: 344-5. [PubMed: 17504289](Two cases with liver injury and jaundice after 5-FU therapy of colon cancer: 66 year old man developed jaundice 6 weeks after finishing 30 weekly courses of 5-FU and leucovorin [bilirubin 30.7 mg/dL, ALT 866 U/L, Alk P 170 U/L], resolving within 4 months; 62 year old man developed jaundice 2 weeks after an initial 5 day cycle of daily 5-FU and leucovorin [bilirubin 3.7 mg/dL, ALT 433 U/L, Alk P 425 U/L], resolving within 4 months of stopping chemotherapy).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. PubMed Citation (Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 2 attributed to antineoplastic agents, 1 to melphalan and 1 to gemtuzumab, but none to fluorouracil or other antimetabolites).
- Wolf PS, Park JO, Bao F, Allen PJ, DeMatteo RP, Fong Y, Jarnagin WR, et al. Preoperative chemotherapy and the risk of hepatotoxicity and morbidity after liver resection for metastatic colorectal cancer: a single institution experience. J Am Coll Surg 2013; 216: 41-9. [PubMed: 23041049](Among 250 patients who received preoperative chemotherapy with fluorouracil and other agents before liver resection for metastatic colorectal cancer, steatosis in the non-tumorous liver was associated with irinotecan [46% vs 31%], higher BMI [44% vs 18%] and diabetes [52% vs 32%], and major liver complications were associated with the size of the resection not chemotherapy regimen).
- Vincenzi B, Imperatori M, Picardi A, Vespasiani Gentilucci U, Gallo P, Fausti V, Spalato Ceruso M, et al. Liver toxicity in colorectal cancer patients treated with first-line FOLFIRI-containing regimen: a single institution experience. Expert Rev Anticancer Ther 2015; 15: 971-6. [PubMed: 26112080](Among 156 patients with metastatic colorectal cancer undergoing chemotherapy with folinic acid, fluorouracil and irinotecan [FOLFIRI], ALT, AST and Alk P elevations were frequent in a "mixed" pattern causing delay in courses in 16%, decrease in doses in 8% and discontinuation in 2%).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. PubMed Citation. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 [5.5%] were attributed to antineoplastic agents, but none to use of fluorouracil).
- Kusano M, Honda M, Okabayashi K, Akimaru K, Kino S, Tsuji Y, Watanabe M, et al. Randomized controlled Phase III study comparing hepatic arterial infusion with systemic chemotherapy after curative resection for liver metastasis of colorectal carcinoma: JFMC 29-0003. J Cancer Res Ther 2017; 13: 84-90. [PubMed: 28508838](Among 91 patients with metastatic colorectal cancer who received fluorouracil intravenously or by intrahepatic arterial infusion, adverse event rates were similar, but rates of treatment interruption and discontinuation were higher with the direct hepatic artery infusions; no mention of ALT elevations or hepatotoxicity).
- Guo JH, Zhang HY, Gao S, Zhang PJ, Li XT, Chen H, Wang XD, et al. Hepatic artery infusion with raltitrexed or 5-fluorouracil for colorectal cancer liver metastasis. World J Gastroenterol 2017; 23: 1406-11. [PMC free article: PMC5330825] [PubMed: 28293087](Among 24 patients with metastatic colorectal cancer treated with intraarterial fluorouracil and oxaliplatin, median survival was 15 months and enzyme elevations occurred in 79%, which were above 5 times ULN in 29%).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Capecitabine.[LiverTox: Clinical and Researc...]Review Capecitabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Pyrimidine Analogues.[LiverTox: Clinical and Researc...]Review Pyrimidine Analogues.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Gemcitabine.[LiverTox: Clinical and Researc...]Review Gemcitabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Taurine as a protective agent for 5-fluorouracil-induced hepatic damage related to oxidative stress.[Pharmazie. 2016]Taurine as a protective agent for 5-fluorouracil-induced hepatic damage related to oxidative stress.Fukuno S, Nagai K, Yoshida S, Suzuki H, Konishi H. Pharmazie. 2016 Sep 1; 71(9):530-532.
- [5-Fluorouracil concentration in various tissues from cancer patients after oral administration of 5-fluorouracil].[Gan To Kagaku Ryoho. 1984][5-Fluorouracil concentration in various tissues from cancer patients after oral administration of 5-fluorouracil].Nakamura T, Hashimoto I, Sawada Y, Mikami J, Yoshimoto M, Nishidai H, Nakanishi Y, Kashi Y. Gan To Kagaku Ryoho. 1984 May; 11(5):1037-48.
- Fluorouracil - LiverToxFluorouracil - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...