NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Cangrelor is an intravenously administered antiplatelet drug that is used at the time of cardiac surgery or percutaneous coronary intervention to decrease the risk of myocardial infarction and maintain artery and stent patency. Cangrelor has not been linked to serum enzyme abnormalities or to clinically apparent liver injury, but its clinical use has been limited.
Background
Cangrelor (kan' grel or) is a non-thienopyridine, reversible inhibitor of adenosine diphosphate (ADP) receptors (P2Y 12) on platelets and is used to decrease the risk of recurrent coronary thromboses in patients who undergo coronary interventions. Activated platelets release ADP which binds to ADP platelet receptors, causing activation of intracellular glycoprotein IIb/IIIA complex which triggers platelet adherence and aggregation. The aggregation of platelets plays an important role in the growth of atheromatous plaques, which can lead to coronary, cerebral and peripheral arterial occlusions. Cangrelor has a rapid onset of action and short-half life, making it an appropriate agent for short term intravenous use. In clinical trials, cangrelor therapy during acute coronary events (unstable angina and myocardial infarction) was shown to decrease the frequency of recurrence of myocardial infarction and stent thromboses. Cangrelor was approved for use in the United States in 2015 and current indications are reduction of recurrent cardiovascular events in patients with acute coronary syndromes. Cangrelor is available in single use vials of 50 mg of lyophilized powder for reconstitution under the commercial name Kengreal. The usual dose regimen is a bolus of 30 µg/kg before the procedure followed by 4 µg/kg/min for at least 2 hours or the duration of the procedure. Side effects are not common, but can include excess bleeding, dyspnea and hypersensitivity reactions.
Hepatotoxicity
In several large clinical trials, serum ALT elevations were no more frequent with cangrelor therapy than with placebo [9% vs 12%] or with comparator arms [6.6% vs 6.8%] and no cases of clinically apparent liver injury with jaundice were reported. In addition, since marketing and release, there have been no published reports of clinically apparent liver injury or jaundice associated with cangrelor therapy and hepatotoxicity is not mentioned in the product label.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Cangrelor, unlike clopidogrel, does not require metabolic activation for its antiplatelet effects. Cangrelor is metabolized in the liver predominantly via CYP 3A4 and it should be used with caution in patients taking CYP 3A4 inhibitors which can increase serum levels, or CYP 3A4 inducers which can decrease drug levels.
Outcome and Management
There is little evidence that cangrelor can cause liver injury or has any cross sensitivity to other antiplatelet agents.
Drug Class: Antithrombotic Agents, Antiplatelet Agents
Other Drugs in the Subclass, Antiplatelet Agents: Aspirin, Clopidogrel, Dipyridamole, Prasugrel, Ticagrelor, Ticlopidine, Vorapaxar
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cangrelor – Generic, Kengreal®
DRUG CLASS
Antithrombotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Cangrelor | 163706-06-7 | C17-H25-Cl2-F3-N5-O12-P3-S2 |
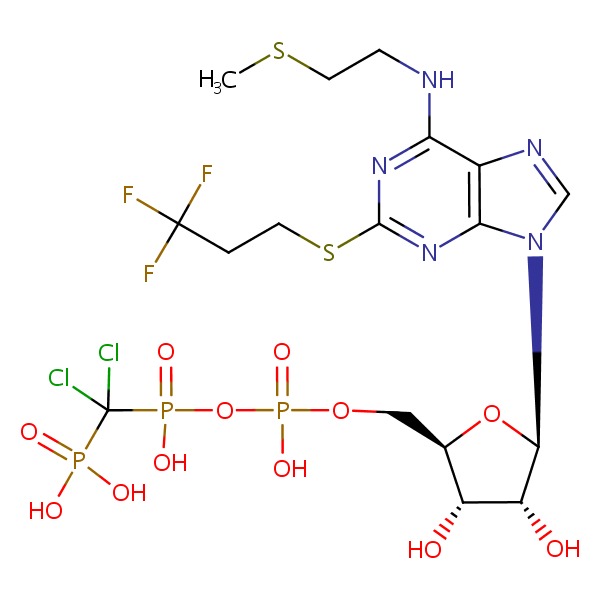 |
ANNOTATED BIBLIOGRAPHY
References updated: 03 October 2017
- Zimmerman HJ. Platelet aggregation inhibitors. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 641-3.(Textbook of hepatotoxicity published in 1999; ticlopidine, but not clopidogrel, prasugrel, cangrelor or ticagrelor is discussed).
- De Marzio DH, Navarro VJ. Antiplatelet agents. Hepatotoxicity of cardiovascular and antidiabetic drugs: antihypertensives. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 527-8.(Review of hepatotoxicity of antiplatelet drugs discusses ticlopidine, clopidogrel and prasugrel, but does not mention ticagrelor or cangrelor).
- Weitz JI. Blood coagulation and anticoagulant, fibrinolytic, and antiplatelet drugs. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 849-76.(Textbook of pharmacology and therapeutics).
- Greenbaum AB, Ohman EM, Gibson CM, Borzak S, Stebbins AL, Lu M, Le May MR, et al. Preliminary experience with intravenous P2Y12 platelet receptor inhibition as an adjunct to reduced-dose alteplase during acute myocardial infarction: results of the Safety, Tolerability and Effect on Patency in Acute Myocardial Infarction (STEP-AMI) angiographic trial. Am Heart J 2007; 154: 702-9. [PubMed: 17892995](Among 92 patients undergoing coronary artery fibrolytic therapy given alteplase, cangrelor or both, patency was more frequent with alteplase [50%] and alteplase and cangrelor [55%] than cangrelor alone [18%]; no mention of ALT elevations or hepatotoxicity).
- Angiolillo DJ, Firstenberg MS, Price MJ, Tummala PE, Hutyra M, Welsby IJ, Voeltz MD, et al.; BRIDGE Investigators. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA 2012; 307: 265-74. [PMC free article: PMC3774162] [PubMed: 22253393](Among 210 patients undergoing coronary artery bypass surgery treated with cangrelor as a bridge from oral antiplatelet therapy, ALT elevations above 3 times ULN were similar in the group [2.3% vs 3.8%]).
- Steg PG, Bhatt DL, Hamm CW, Stone GW, Gibson CM, Mahaffey KW, Leonardi S, et al.; CHAMPION Investigators. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet 2013; 382 (9909): 1981-92. [PubMed: 24011551](A pooled analysis of 3 controlled trials of cangrelor therapy during percutaneous coronary interventions in 24,910 patients, found a reduction in thrombotic events with cangrelor therapy despite similar rates of serious adverse events in both groups [2.2% in each], the only side effect more frequent with cangrelor being dyspnea [1.1% vs 0.4%]).
- Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, Price MJ, et al.; CHAMPION PHOENIX Investigators. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med 2013; 368: 1303-13. [PubMed: 23473369](Among 11,145 patients undergoing percutaneous coronary interventions who received cangrelor or clopidogrel, thrombotic events within 48 hours were less frequent with cangrelor [4.7% vs 5.9%] and adverse event rates were low; no mention of ALT elevations or hepatotoxicity).
- Antithrombotic drugs. Med Lett Drugs Ther 2014; 56: 103-9. PubMed Citation. [PubMed: 25337986](Guidelines on use of antiplatelet agents including aspirin, clopidogrel, prasugrel and ticagrelor does not discuss cangrelor).
- Abtan J, Steg PG, Stone GW, Mahaffey KW, Gibson CM, Hamm CW, Price MJ, et al.; CHAMPION PHOENIX Investigators. Efficacy and safety of cangrelor in preventing periprocedural complications in patients with stable angina and acute coronary syndromes undergoing percutaneous coronary intervention: The CHAMPION PHOENIX Trial. JACC Cardiovasc Interv 2016; 9: 1905-13. [PubMed: 27659566](Subgroup analysis of the CHAMPION trial [Bhatt 2013] demonstrated a consistent beneficial effect of cangrelor compared to clopidogrel in patients with stable angina and in those with an acute coronary syndrome; no discussion of adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Platelet Inhibition With Cangrelor and Crushed Ticagrelor in Patients With ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention.[Circulation. 2019]Platelet Inhibition With Cangrelor and Crushed Ticagrelor in Patients With ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention.Franchi F, Rollini F, Rivas A, Wali M, Briceno M, Agarwal M, Shaikh Z, Nawaz A, Silva G, Been L, et al. Circulation. 2019 Apr 2; 139(14):1661-1670.
- Ischemic Events Occur Early in Patients Undergoing Percutaneous Coronary Intervention and Are Reduced With Cangrelor: Findings From CHAMPION PHOENIX.[Circ Cardiovasc Interv. 2022]Ischemic Events Occur Early in Patients Undergoing Percutaneous Coronary Intervention and Are Reduced With Cangrelor: Findings From CHAMPION PHOENIX.Cavender MA, Harrington RA, Stone GW, Steg PG, Gibson CM, Hamm CW, Price MJ, Lopes RD, Leonardi S, Deliargyris EN, et al. Circ Cardiovasc Interv. 2022 Jan; 15(1):e010390. Epub 2021 Dec 17.
- Outcomes with cangrelor versus clopidogrel on a background of bivalirudin: insights from the CHAMPION PHOENIX (A Clinical Trial Comparing Cangrelor to Clopidogrel Standard Therapy in Subjects Who Require Percutaneous Coronary Intervention [PCI]).[JACC Cardiovasc Interv. 2015]Outcomes with cangrelor versus clopidogrel on a background of bivalirudin: insights from the CHAMPION PHOENIX (A Clinical Trial Comparing Cangrelor to Clopidogrel Standard Therapy in Subjects Who Require Percutaneous Coronary Intervention [PCI]).White HD, Bhatt DL, Gibson CM, Hamm CW, Mahaffey KW, Price MJ, Steg PG, Stone GW, Cortese B, Wilensky M, et al. JACC Cardiovasc Interv. 2015 Mar; 8(3):424-433. Epub 2015 Feb 18.
- Review Cangrelor in Percutaneous Coronary Intervention: Current Status and Perspectives.[J Cardiovasc Pharmacol Ther. 2...]Review Cangrelor in Percutaneous Coronary Intervention: Current Status and Perspectives.Alexopoulos D, Pappas C, Sfantou D, Lekakis J. J Cardiovasc Pharmacol Ther. 2018 Jan; 23(1):13-22. Epub 2017 Jun 15.
- Review Cangrelor versus clopidogrel in percutaneous coronary intervention: a systematic review and meta-analysis.[EuroIntervention. 2014]Review Cangrelor versus clopidogrel in percutaneous coronary intervention: a systematic review and meta-analysis.Pandit A, Aryal MR, Aryal Pandit A, Jalota L, Hakim FA, Mookadam F, Lee HR, Tleyjeh IM. EuroIntervention. 2014 Mar 20; 9(11):1350-8.
- Cangrelor - LiverToxCangrelor - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...