NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Prasugrel is an inhibitor of platelet aggregation that is used to decrease the risk of myocardial infarction and stroke in patients with acute coronary syndromes. Prasugrel has been linked to mild and transient serum enzyme elevations during therapy and to rare instances of hypersensitivity reactions accompanied by mild liver injury.
Background
Prasugrel (pra' soo grel) is a thienopyridine inhibitor of adenosine diphosphate (ADP) receptors (P2Y12) on platelets, and is used as an anticoagulant to decrease the risk of recurrent coronary thromboses in patients who undergo interventions during an acute coronary syndrome. Activated platelets release ADP which binds to ADP platelet receptors, causing activation of the intracellular glycoprotein IIb/IIIA complex which triggers platelet adherence and aggregation. The aggregation of platelets plays an important role in the growth of atheromatous plaques, which can lead to coronary, cerebral and peripheral arterial occlusions. Prasugrel is an irreversible inhibitor of the P2Y12 receptor and its effects last for the life time of the platelet (7 to 10 days). In clinical trials, prasugrel therapy during acute coronary events (unstable angina and myocardial infarction) was equivalent or slightly better than clopidogrel in decreasing the frequency of recurrence of myocardial infarction and stent thrombosis. Prasugrel was approved for use in the United States in 2009. Current indications are reduction of recurrent cardiovascular events in patients with acute coronary syndromes who are to be managed with percutaneous coronary intervention. Prasugrel is available in 5 and 10 mg tablets generically and under the commercial name Effient. The usual oral dose is a loading dose of 60 mg followed by 10 mg daily in combination with aspirin. The most common side effect is bleeding (usually epistaxis); other side effects are not common, but can include headache, dizziness, fatigue, gastrointestinal upset, nausea, arthralgias and rash. Rare, but more severe adverse events include major episodes of bleeding and hypersensitivity reactions including anaphylaxis.
Hepatotoxicity
Prasugrel is associated with low rates of serum enzyme elevations during therapy, which are similar to those with clopidogrel. In premarketing studies, no instances of clinically apparent liver injury were reported. Since marketing and release, there has been several reports of liver injury attributed to prasugrel often as a part of a hypersensitivity reaction. The onset was within a few weeks of switching from clopidogrel to prasugrel, and was accompanied by mild-to-moderate ALT and GGT elevations that resolved rapidly once prasugrel was replaced by clopidogrel. The liver injury was overshadowed by features of a hypersensitivity syndrome with fever, eosinophilia and diarrhea, which also reversed rapidly with stopping prasugrel. Autoantibodies were not reported. There has also been one report of a case of hepatocellular jaundice that resolved only after stopping prasugrel. There have been no reports of chronic injury or acute liver failure reported with prasugrel, although such instances have been reported with clopidogrel and ticlopidine.
Likelihood score: D (possible rate cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of prasugrel hepatotoxicity is not known, but is likely to be immunologically mediated and due to hypersensitivity. Prasugrel requires metabolic activation for its antiplatelet effects, which occurs in the liver primarily via the cytochrome P450 system, CYP 3A4 and 2B6. Inhibitors of CYP 3A4 (such as clarithromycin or itraconazole) may result in higher levels of prasugrel and antiplatelet activity, whereas inducers of CYP 3A4 (such as rifampin or phenytoin) may lower levels and result in less of an antiplatelet response.
Outcome and Management
The severity of liver injury associated with prasugrel has been mild and rapidly reversible with stopping therapy. In one instance, switching anticoagulant therapy to clopidogrel was tolerated without recurrence. Rechallenge should be avoided.
Drug Class: Antithrombotic Agents, Antiplatelet Agents
Other Drugs in the Subclass, Antiplatelet Agents: Aspirin, Cangrelor, Clopidogrel, Dipyridamole, Ticagrelor, Ticlopidine, Vorapaxar
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Prasugrel – Generic, Effient®
DRUG CLASS
Antithrombotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Prasugrel | 150322-43-3 | C20-H20-F-N-O3-S |
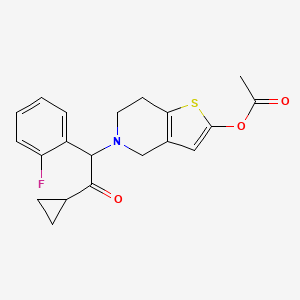
|
ANNOTATED BIBLIOGRAPHY
References updated: 15 September 2020
- Zimmerman HJ. Platelet aggregation inhibitors. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 641-3.(Textbook of hepatotoxicity published in 1999; ticlopidine, but not clopidogrel or prasugrel is discussed).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs: antihypertensives. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 528.(Review of hepatotoxicity of antiplatelet drugs mentions that prasugrel is a recently approved inhibitor of platelet aggregation and has yet to be linked to cases of clinically apparent liver injury).
- Hogg K, Weitz JI. Blood coagulation and anticoagulant, fibrinolytic, and antiplatelet drugs. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 849-76.(Textbook of pharmacology and therapeutics).
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, et al. TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. [PubMed: 17982182](Controlled trial comparing prasugrel to clopidogrel in 13,608 patients with acute coronary syndromes with planned intervention found similar rates of efficacy, but higher rates of major bleeding with prasugrel [2.4% vs 1.8%]; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to antiplatelet agents).
- Scott DM, Norwood RM, Parra D. P2Y12 inhibitors in cardiovascular disease: focus on prasugrel. Ann Pharmacother. 2009;43:64–76. [PubMed: 19050170](Review of efficacy and safety of prasugrel in comparison to other antiplatelet agents; mentions that: "..elevated liver function tests could occur with prasugrel treatment").
- Prasugrel (Effient) vs. clopidogrel (Plavix). Med Lett Drugs Ther. 2009;51:69–70. [PubMed: 19738549](Concise review of pharmacology, efficacy and safety of prasugrel in relation to clopidogrel, mentions the main adverse events is bleeding which is more common with prasugrel; no mention of hepatotoxicity or ALT elevations).
- Mohammad RA, Goldberg T, Dorsch MP, Cheng JW. Antiplatelet therapy after placement of a drug-eluting stent: a review of efficacy and safety studies. Clin Ther. 2010;32:2265–81. [PubMed: 21353100](Systematic review of studies of antiplatelet therapy after coronary stenting discussed bleeding complications only, no mention of ALT elevations or hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were due to antiplatelet medications).
- Antithrombotic drugs. Treat Guidel Med Lett. 2011;9(110):61–6. [PubMed: 21941228](Guidelines on the use of antiplatelet drugs, including aspirin, clopidogrel, prasugrel, ticagrelor and ticlopidine, mentions that prasugrel appears to be more effective than clopidogrel, but has a greater risk of bleeding; no mention of hepatotoxicity or ALT elevations).
- Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, Cornel JH, et al. TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297–309. [PubMed: 22920930](Among 7243 patients with unstable angina or myocardial infarction treated with prasugrel or clopidogrel for up to 30 months, both efficacy and safety were similar between the two groups; no mention of ALT elevations or hepatotoxicity).
- Fernández-Ruiz M, Carbonell-Porras A, García-Reyne A, López-Medrano F. Management of a hypersensitivity reaction to thienopyridines: prasugrel-induced fever and hepatitis resolved after switching to clopidogrel. Rev Esp Cardiol. 2012;65:773–4. [PubMed: 22377199](40 year old woman developed fever, eosinophilia, diarrhea and liver enzyme elevations starting a few weeks after switching from clopidogrel to prasugrel [peak ALT 155 U/L, GGT 723 U/L, 10% eosinophils], resolving rapidly upon switching back).
- Serebruany VL, Kipshidze N, Pershukov IV, Kuliczkowski W, Carnes J, Atar D. Fatal sepsis and systemic inflammatory response syndrome after off-label prasugrel: a case report. Am J Ther. 2014;21:e229–33. [PubMed: 23665886](65 year old man developed hypersensitivity reaction with high fever, confusion and rash 6 days after switching from clopidogrel to prasugrel, with subsequent multiorgan failure and death from sepsis by 16 days; few details given).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to prasugrel).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one was attributed to prasugrel: a 49 year old man who developed jaundice 3 months after starting prasugrel, atorvastatin and ranolazine that did not improve after stopping atorvastatin and ranolazine, but did after stopping prasugrel 6 weeks later).
- Schüpke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wöhrle J. Richardt Get al.; ISAR-REACT 5 Trial Investigators. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2019;381:1524–34. [PubMed: 31475799](Among 4018 patients with acute coronary syndromes treated with ticagrelor or prasugrel for one year, major cardiovascular events were less with prasugrel [6.9% vs 9.3%], but major bleeding event rates were similar [4.8% vs 5.4%]; no mention of ALT elevations or hepatotoxicity).
- Nakamura M, Kitazono T, Kozuma K, Sekine T, Nakamura S, Shiosakai K, Iizuka T. Prasugrel for Japanese patients with ischemic heart disease in long-term clinical practice (PRASFIT-Practice II)- 1-year follow-up results of a postmarketing observational study. Circ J. 2019;84(1):101–8. [PubMed: 31748446](Among 4155 Japanese patients followed in a postmarketing observation study after starting prasugrel, major bleeding episodes occurred in 1%, any bleeding episode in 5.5% and 0.2% developed abnormal liver tests; no details provide).
- Tarantini G, Mojoli M, Varbella F, Caporale R, Rigattieri S, Andò G, Cirillo P, et al.; DUBIUS Investigators, on behalf of the Italian Society of Interventional Cardiology (SICI-GISE). Timing of oral P2Y inhibitor administration in non-ST elevation acute coronary syndrome. J Am Coll Cardiol 2020: S0735-1097(20)36444-5. Epub ahead of print.(Among 1449 patients undergoing coronary angiography treated with prasugrel or ticagrelor, rates of a combined clinical efficacy endpoint were similar in the two groups; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- The efficacy and safety of prasugrel with and without a glycoprotein IIb/IIIa inhibitor in patients with acute coronary syndromes undergoing percutaneous intervention: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) analysis.[J Am Coll Cardiol. 2009]The efficacy and safety of prasugrel with and without a glycoprotein IIb/IIIa inhibitor in patients with acute coronary syndromes undergoing percutaneous intervention: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) analysis.O'Donoghue M, Antman EM, Braunwald E, Murphy SA, Steg PG, Finkelstein A, Penny WF, Fridrich V, McCabe CH, Sabatine MS, et al. J Am Coll Cardiol. 2009 Aug 18; 54(8):678-85.
- Discharge aspirin dose and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel: an analysis from the TRITON-TIMI 38 study (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38).[J Am Coll Cardiol. 2014]Discharge aspirin dose and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel: an analysis from the TRITON-TIMI 38 study (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38).Kohli P, Udell JA, Murphy SA, Cannon CP, Antman EM, Braunwald E, Wiviott SD. J Am Coll Cardiol. 2014 Jan 28; 63(3):225-32. Epub 2013 Oct 16.
- Elderly patients with acute coronary syndromes managed without revascularization: insights into the safety of long-term dual antiplatelet therapy with reduced-dose prasugrel versus standard-dose clopidogrel.[Circulation. 2013]Elderly patients with acute coronary syndromes managed without revascularization: insights into the safety of long-term dual antiplatelet therapy with reduced-dose prasugrel versus standard-dose clopidogrel.Roe MT, Goodman SG, Ohman EM, Stevens SR, Hochman JS, Gottlieb S, Martinez F, Dalby AJ, Boden WE, White HD, et al. Circulation. 2013 Aug 20; 128(8):823-33. Epub 2013 Jul 12.
- Review Prasugrel: a review of its use in patients with acute coronary syndromes undergoing percutaneous coronary intervention.[Drugs. 2009]Review Prasugrel: a review of its use in patients with acute coronary syndromes undergoing percutaneous coronary intervention.Duggan ST, Keating GM. Drugs. 2009 Aug 20; 69(12):1707-26.
- Review Review of prasugrel for the secondary prevention of atherothrombosis.[J Manag Care Pharm. 2009]Review Review of prasugrel for the secondary prevention of atherothrombosis.Spinler SA, Rees C. J Manag Care Pharm. 2009 Jun; 15(5):383-95.
- Prasugrel - LiverToxPrasugrel - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...