NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Aspirin or acetylsalicylic acid is perhaps the most commonly used analgesic and antipyretic medication worldwide, having been in clinical use for over 100 years. Aspirin can cause several forms of liver injury: in high doses, aspirin can cause moderate to marked serum aminotransferase elevations occasionally with jaundice or signs of liver dysfunction, and in lower doses in susceptible children with a febrile illness aspirin can lead to Reye syndrome.
Background
Aspirin is a salicylate, but technically is also a nonsteroidal antiinflammatory drug (NSAID). Like the NSAIDs, salicylates are inhibitors of tissue cyclooxygenases (Cox-1 and -2) which cause a decrease in synthesis of proinflammatory prostaglandins, potent mediators of pain and inflammation. In distinction to other NSAIDs, however, aspirin is a noncompetitive and irreversible inhibitor of Cox-1, so that its effects are longer lasting and less easily reversed than those of typical NSAIDs. Aspirin has potent effects in inhibiting platelet function that lasts for the lifetime of the platelet. Aspirin’s potent and lasting effects on Cox-1 in gastric epithelial cells account for its frequent gastric side effects and association with peptic ulcer disease and gastrointestinal bleeding. Aspirin is indicated for the treatment of mild to moderate pain from headaches, colds, arthritis, menstrual periods, toothaches and joint and muscle aches caused by trauma, osteoarthritis, or rheumatoid arthritis. Higher, more continuous doses of aspirin are effective in therapy of juvenile rheumatoid arthritis, systemic lupus erythematosus, rheumatoid arthritis, acute rheumatic fever and Kawasaki disease. Aspirin has antipyretic effects and can be used for management of fever, but should not be used in children or adolescents because of its potential to cause Reye syndrome. In low daily doses (81 mg), aspirin is used to decrease the risk of coronary and cerebrovascular disease and reocclusion after coronary revascularization or stent placement. Aspirin became clinically available in the United States in the early part of the 20th century and is currently widely used as an over-the-counter medication. It is available in multiple generic formulations, either alone or in combination with other pain relievers, antacids, or cough and cold medications. Aspirin is typically taken in doses of 330 to 660 mg every 4 to 6 hours. The dose used for antiplatelet effects in prevention of complications of atherosclerosis is 81 mg once daily. Common brand names for aspirin alone or in combination with other agents include Bayer’s Aspirin, Alka Seltzer, Anacin, Ascriptin, Aspergum, BC Powder, Bufferin, Ecotrin, Excedrin and Stanback.
Hepatotoxicity
Patients on long term, moderate-to-high dose aspirin therapy frequently have elevations in serum ALT levels. With high doses, ALT elevations are common and can be marked and associated with mild increases in alkaline phosphatase and bilirubin. The more dramatic examples of aspirin hepatotoxicity usually occur with doses of 1,800 to 3,200 mg daily (>100 mg/kg) and with salicylate levels of greater than 25 mg/dL, but mild-to-moderate ALT elevations occur with even lower doses and lower serum levels. These abnormalities resolve rapidly with discontinuation of aspirin, but instances of resolution despite continuation of aspirin in the same or lower doses (adaptation) have also been described. The hepatotoxicity of aspirin is usually mild and asymptomatic, although with higher doses symptoms of nausea, anorexia and abdominal pain and even encephalopathy with signs of hepatic dysfunction (hyperammonemia and coagulopathy) can occur. Bilirubin elevations are usually mild or absent. Mild eosinophilia may accompany the enzyme elevations, but rash, fever and other allergic manifestations are rare. Liver biopsy histology generally shows minimal injury despite the height of the enzyme elevations; electron microscopy may reveal fat and mitochondrial abnormalities. Aspirin can often be continued in lower doses safely.
Likelihood score: A[HD] (well known cause of clinically apparent liver injury when given in high doses).
Reye Syndrome
A special form of aspirin hepatotoxicity is Reye Syndrome, the development of lactic acidosis, microvesicular fat and hepatic dysfunction with encephalopathy and coma. Serum aminotransferase levels are usually markedly increased while serum bilirubin is minimally or only moderated elevated despite signs of hepatic failure such as hyperammonemia and encephalopathy. Reye syndrome usually occurs in children or young adults developing a few days to a week after a prodromal febrile illness, typically influenza B or varicella. It is often rapidly fatal, but in milder cases recovery is rapid. Reye syndrome was first reported in Australia in 1963, but subsequently was reported from around the world with increasing frequency and peaking in incidence in the 1970s and 1980s. Subsequently, case reports followed by careful epidemiological surveys linked the occurrence of Reye syndrome to receipt of aspirin during the prodromal viral illness. With medical recognition of this association, followed by wide scale public warnings, the use of aspirin in children with fever decreased markedly and the frequency of Reye syndrome fell dramatically. In the United States, reported cases of Reye syndrome fell from more than 500 cases per year before 1986 to less 2 cases per year thereafter. Occasional rare case reports of Reye syndrome still appear. Reye syndrome can also occur in adults.
Mechanism of Injury
The association of hepatotoxicity with use of high doses of aspirin, short latency and linkage to high serum levels of salicylates suggest that aspirin is a direct, intrinsic hepatotoxin. Patients with severe hepatic reactions to aspirin can safely take acetaminophen or other NSAIDs, and usually tolerate lower doses of aspirin without problems. In the case of Reye syndrome, aspirin has been shown to inhibit mitochondrial function and the combination of a systemic viral illness with drug induced mitochondrial dysfunction is thought to underlie the pathogenesis of Reye syndrome. Other drugs that inhibit mitochondrial function (amiodarone, valproate, nucleoside analogues) can cause a similar clinical syndrome. The mitochondrial failure is manifested by Lactic Acidosis, acute microvesicular Steatosis and Hepatic dysfunction (LASH).
Outcome and Management
Liver injury from high doses of aspirin is usually mild and self-limited. Symptoms attributable to liver injury (as opposed to the other side effects of aspirin itself) are common but generally nonspecific and mild. Typically, ALT elevations fall to normal within days of stopping. No convincing cases of acute liver failure or chronic liver injury or chronic vanishing bile duct syndrome due to aspirin have been published. Reye syndrome induced by aspirin, on the other hand, is a serious and potentially life threatening condition that should be managed with emergency intensive care. The mitochondrial failure of Reye syndrome is rapidly reversible and the major focus of management should be clinical support during the acute phase. Infusions of 20% glucose may help sustain hepatic and brain function during the temporary mitochondrial failure. Recurrence of Reye syndrome has been reported in children who have recovered and were then treated again with aspirin during an acute febrile illness. Children who require long term therapy with aspirin or other mitochondrial inhibitors should receive influenza and varicella vaccine and parents should be alert to the signs and symptoms of Reye syndrome.
Drug Class: Antithrombotic Agents, Antiplatelet Agents, Salicylates
Other Drugs in the Subclass, Antiplatelet Agents: Cangrelor, Clopidogrel, Dipyridamole, Prasugrel, Ticagrelor, Ticlopidine, Vorapaxar
Other Drugs in the Salicylates Class: Diflunisal, Salsalate, Trisalicylate
CASE REPORTS
Case 1. Acute enzyme elevations due to high dose aspirin therapy.
[Modified from: Wolfe JD, Metzger AL, Goldstein RC. Aspirin hepatitis. Ann Intern Med 1974; 80: 74-6. PubMed Citation]
A 20 year old woman with systemic lupus erythematosus was treated with aspirin in doses of 4.8 grams per day. Her arthralgias improved, but 5 days after starting she noted the onset of tinnitus, anorexia, nausea, and abdominal discomfort. A serum salicylate level was 45 mg/dL (high) and serum enzyme levels were elevated (Table). Her liver was tender, but she was not jaundiced. Aspirin was stopped and her symptoms and enzyme abnormalities resolved within a few days. Aspirin was subsequently restarted at the same dose. She developed symptoms within 2 days and serum ALT levels began to rise. A liver biopsy showed spotty necrosis and inflammation without fat or chronic liver disease. Aspirin was stopped and she was later treated successfully with corticosteroids.
Key Points
| Medication: | Aspirin (4.8 g daily) |
|---|---|
| Pattern: | Mixed (R=2.5) |
| Severity: | 1+ (enzyme elevations without jaundice) |
| Latency: | 5 days initially, 2 days on rechallenge |
| Recovery: | Rapid (7-10 days) |
| Other medications: | None mentioned |
Laboratory Values
| Days After Starting | Days After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | 30 | 75 | Normal | Aspirin started: 4.8 g/day | |
| 5 | 0 | 168 | 197 | Symptoms: salicylate level 45 mg/dL, aspirin stopped | |
| 7 | 2 | 175 | 205 | ||
| 10 | 5 | 55 | 180 | ||
| 12 | 7 | 30 | 96 | ||
| 18 (0) | 13 | 30 | 35 | Aspirin restarted at 4.8 g/day | |
| 20(2) | 100 | 105 | |||
| 21(3) | 0 | 300 | 205 | Normal | Liver biopsy: aspirin stopped |
| 22(4) | 1 | 440 | 400 | ||
| 23(5) | 2 | 360 | 370 | ||
| 24(6) | 3 | 230 | 360 | ||
| 25(7) | 4 | 110 | 260 | Discharged on prednisone | |
| 33(15) | 12 | 20 | 55 | ||
| 53(35) | 32 | 25 | 40 | ||
| Normal Values | <40 | <115 | <1.2 | ||
Comment
A very convincing case of aspirin induced hepatotoxicity. The onset of liver injury was within a few days of starting high doses of aspirin. The patient was mildly symptomatic but not jaundiced. There were also changes in alkaline phosphatase levels. Toxicity appeared to correlate with serum salicylate levels. Recovery was rapid once ASA was stopped and recurred when aspirin was restarted. Lower doses may have been tolerated without toxicity but were considered subtherapeutic for management of lupus. Liver biopsy showed minor nonspecific changes.
Case 2. Reye syndrome in a child with juvenile rheumatoid arthritis.
[Modified from: Norman MG, Lowden JA, Hill DE, Bannayne RM. Encephalopathy and fatty degeneration of the viscera in childhood: II. Report of a case with isolation of influenza B virus. Canad Med Assoc J 1968; 99: 522-6. PubMed Citation].
A 9 year old girl with juvenile rheumatoid arthritis on salicylates developed nausea, vomiting and drowsiness and was admitted to hospital for suspected salicylate intoxication. However, blood salicylate levels were within the therapeutic range (17.5/mg/dL) and she developed progressive restlessness, confusion and ultimately coma. She had acidosis and hypoglycemia and was treated with intravenous fluids. Serum AST levels were normal on admission but rose to 696 U/L, which led to an emergency liver biopsy that showed microvesicular fatty change with no inflammation, fibrosis or architectural distortion. The liver was devoid of glycogen. Despite supportive care and artificial ventilation she developed progressive coma, cerebral edema and died 72 hours after admission which was 5 days after initial symptoms of vomiting. Autopsy showed cerebral edema and steatosis in the liver with both macro- and microvesicular fat. Although there was no antecedent history of upper respiratory illness, she had complained of headaches for 2 weeks before onset and influenza B virus was isolated from the liver biopsy and from multiple tissues at autopsy.
Key Points
| Medication: | Aspirin (salicylate levels maintained at 20-25 mg/dL) |
|---|---|
| Pattern: | Hepatocellular (acute fatty liver) (R=33) |
| Severity: | 5+ (death) |
| Latency: | 2 months from initiation of aspirin use, 1 day after onset of vomiting |
| Recovery: | None |
| Other medications: | Vitamins, throat lozenges and proprietary laxative |
Laboratory Values
| Days After Stopping | AST (U/L) | Alk P (KA U/L) | Arterial pH | Glucose (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | 7.25 | Serum salicylate levels 17.5 mg/dL | |||
| 1 | 35 | 27 | 6.80 | 25 | IV fluids initiated, bilirubin 1.2 mg/dL |
| 2 | 696 | 12 | 7.51 | 155 | Ventilatory support, liver biopsy |
| 3 | 475 | 7.43 | >200 | ||
| 4 | 4 | Death from cerebral edema | |||
| Normal | <35 | <20 | 7.40 | 70-115 |
Comment
A well described case of Reye syndrome arising in a child on chronic salicylate therapy for juvenile rheumatoid arthritis. This case was described before epidemiological data linked Reye syndrome with influenza B and aspirin and is strikingly prescient. The initial symptoms were probably due to lactic acidosis, and AST levels rose shortly thereafter. In cases with recovery, aminotransferase levels fall as rapidly as they had risen. Initially, liver tissue shows microvesicular fat and absence of glycogen as the liver cell uses glycolysis to compensate for the lack of ATP produced by mitochondria. When this mechanism fails, lactic acid levels rise and hepatocytes release enzymes triggered by apoptosis from mitochondrial and cell functional failure. These processes occur rapidly, even before aminotransferase and bilirubin levels rise (the latter being a product of hepatocellular failure).
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Acetylsalicylic Acid, Aspirin – Generic, Bayer Aspirin®
DRUG CLASS
Antithrombotic Agents, Antiinflammatory Agents, Salicylates
COMPLETE LABELING (not available)
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
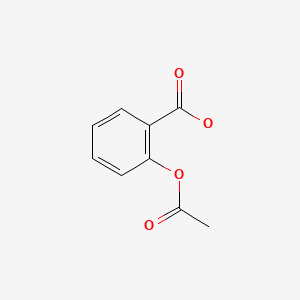
| Aspirin | 50-78-2 | C9-H8-O4 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 27 July 2017
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. Chapter 19: The NSAIDS. In Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott Williams & Williams, 1999, 599-602.(Review of hepatotoxicity of aspirin published in 1999 mentions two different forms of injury; the development of high ALT elevations with high doses of aspirin and Reye syndrome arising days to weeks after aspirin use in children with febrile illnesses).
- Grossner T, Smyth EM, Fitzgerald GA. Anti-inflammatory, antipyretic, and analgesic agents: pharmacotherapy of gout. In, Brunton LL, Chabner BA, Knollman BC. Goodman & Gilman’s The pharmacological basis of therapeutics, 12th ed. New York: McGraw-Hill, 2011. p. 959-1004.(Textbook of pharmacology and therapeutics).
- Manso C, Taranta A, Nydick I. Effect of aspirin administration on serum glutamic oxaloacetic and glutamic pyruvic transaminases in children. Proc Soc Exp Biol Med. 1956;93:84–8. [PubMed: 13370585](Study shortly after development of ALT and AST as tests for liver injury in 23 children convalescing from acute rheumatic fever, showed normal ALT levels before, but rises to 70-900 U/L after 1-2 weeks of salicylates in ~50% of children, rapid recovery).
- Reye RD, Morgan G, Baral J. Encephalopathy and fatty degeneration of the viscera. A disease entity in childhood. Lancet. 1963;2:749–52. [PubMed: 14055046](Initial report of Reye syndrome: 21 children [<1 to 8 years old] seen at Royal Alexandra Hospital, Sydney Australia between 1951-62 with syndrome of upper respiratory illness for 1-3 days followed by abrupt deterioration with severe vomiting, agitation, stupor, convulsions and coma; hyperpnea and liver enlargement common; hypoglycemia and renal insufficiency common, ALT and AST raised and protime prolonged in those tested, autopsy in 17 cases showed cerebral edema but not herniation, enlarged liver with fatty change, but no necrosis or inflammation and no glycogen; may respond to glucose infusions; no toxin identified; mentions recent case reports by Curry and Anderson and similarity to Jamaican vomiting sickness; 81% mortality).
- Okumura H, Takayama K, Obayashi K, Ichikawa T, Aramaki T. Nippon Rinsho. 1965;23:1633–6. [Chronic toxic hepatitis caused by aspirin] Japanese. [PubMed: 5895147](Article in Japanese: case report followed by prospective study of 15 patients given aspirin [2 g/day] for 4 weeks with monitoring of liver tests: ALT levels rose in 5 to as high as 150 U/L with no change in bilirubin or BSP retention; occasional rise in Alk P levels).
- Norman MG. Encephalopathy and fatty degeneration of the viscera in childhood: I. Review of cases at the Hospital for Sick Children, Toronto (1954-1966). Can Med Assoc J. 1968;99:522–6. [PMC free article: PMC1947921] [PubMed: 5696234](21 cases, prodrome of 4 days of upper respiratory illness, followed by vomiting, confusion, convulsions and quiet drift into coma, 50% mortality within 24 hours; hypoglycemia and acidosis frequent, AST 700-1400 U/L, histology showed small fat droplet change).
- Norman MG, Lowden JA, Hill DE, Bannayne RM. Encephalopathy and fatty degeneration of the viscera in childhood: II. Report of a case with isolation of influenza B virus. Can Med Assoc J. 1968;99:549–54. [PMC free article: PMC1947925] [PubMed: 4300792](Case report of child with juvenile rheumatoid arthritis on salicylates who developed Reye syndrome in whom influenza B virus was isolated from liver biopsy and autopsy tissue; AST initially normal [35 U/L] when presenting with acidosis and hyperventilation, later rising to 696 U/L just before death, 5 days after onset: Case 2).
- Russell AS, Sturge RA, Smith MA. Serum transaminases during salicylate therapy. Br Med J. 1971;2:428–9. [PMC free article: PMC1796143] [PubMed: 5576002](Nine of 33 children with chronic aspirin therapy developed high ALT levels, almost all with salicylate levels above 35 mg/dL, mild Alk P elevations in 3 patients).
- Lee-Jones M. Serum transaminases during salicylate therapy. Br Med J. 1971;2:772–3. [PMC free article: PMC1796312] [PubMed: 5090787](Letter in response to Russell [1971], found AST elevations in only 1 of 16 adults with acute salicylate overdose and serum levels >35 mg/dL).
- Iancu T. Serum transaminases and salicylate therapy. Br Med J. 1972;2:167. [PMC free article: PMC1787971] [PubMed: 5017315](Normal ALT and AST levels were found in 41 children with rheumatic fever on admission; but 10 of 14 receiving aspirin had ALT elevations of 51-300 U/L, usually with salicylate levels >30 mg/dL arising in 10-20 days, rapidly resolving with stopping, normal bilirubin; occasionally raised Alk P level).
- Athreya BH, Gorske AL, Myers AR. Aspirin-induced abnormalities of liver function. Am J Dis Child. 1973;126:638–41. [PubMed: 4745155](Child with juvenile rheumatoid arthritis with elevations in ALT during three courses of aspirin; first exposure, ALT 310 U/L and resolution in 7 days; 2nd exposure, ALT 180 U/L and biopsy showed minimal changes; 3rd exposure, ALT 700 U/L, Alk P 400 U/L, bilirubin 2.4 mg/dL with resolution requiring 60 days).
- Mowat AP. Encephalopathy and fatty degeneration of viscera: Reye's syndrome. Arch Dis Child. 1973;48:411–3. [PMC free article: PMC1648436] [PubMed: 4197287](Early review of the histologic features of Reye syndrome: "The liver at necropsy is swollen and tense, orange to pale yellow in colour, the cut surface being relatively bloodless, greasy, and firm; in liver biopsy specimens fatty infiltration is seen as diffuse small vacuoles most prominent in the periportal areas, but there is massive fatty infiltration in necropsy material").
- Rich RR, Johnson JS. Salicylate hepatotoxicity in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1973;16:1–9. [PubMed: 4692157](6 patients with juvenile rheumatoid arthritis treated at the NIH with high doses of salicylates [>25 mg/dL]; high serum levels associated with nausea and anorexia and ALT elevations [100-1800 U/L] and lesser increases in Alk P and eosinophils [from 90, 94, 70, 310, 400 and 175 /μL to 440, 945, 924, 570, 4900, and 485/μL]).
- Liver injury by salicylates. Br Med J. 1973;2:732. [PMC free article: PMC1589834] [PubMed: 4515688](Editorial discussing results of Rich and Johnson [1973]).
- Gitlin N, Dietrich B, Spektor F. Salicylate hepatitis. A case report. S Afr Med J. 1974;48:1998–2000. [PubMed: 4418777](14 year old with juvenile rheumatoid arthritis given 3 to 10 g/day of Disprin [a salicylate] developed fever, rash and eosinophilia with ALT 700 U/L, bilirubin 2.1 mg/dL, Alk P ~1.5 times ULN; rechallenge caused recurrence in 2 days with 38% eosinophils, biopsy showed hepatitis and stellate fibrosis, recovery in <7 days).
- Iancu T, Elian E. Letter: Aspirin-induced abnormalities of liver function. Am J Dis Child. 1974;128:116–7. [PubMed: 4834996](Letter in response to Athreya [1973] reminding the authors of their publication in BMJ [1972]).
- Koppes GM, Arnett FC. Salicylate hepatotoxicity. Postgrad Med. 1974;56:193–5. [PubMed: 4415672](Young man with suspected juvenile rheumatoid arthritis developed AST 2,200 U/L, protime 16 seconds after 2 weeks of aspirin [5.85 g/day, levels >25 mg/dL], biopsy showed acidophilic bodies and mild inflammation; no symptoms, rapid resolution).
- Prinsloo JG. Letter: Salicylate toxicity. S Afr Med J. 1974;48:2540. [PubMed: 4453933](Letter regarding article by Gitlin [1974], arguing against use of high dose aspirin in children).
- Seaman WE, Ishak KG, Plotz PH. Aspirin-induced hepatotoxicity in patients with systemic lupus erythematosus. Ann Intern Med. 1974;80:1–8. [PubMed: 4810348](3 patients with systemic lupus given ASA found to have asymptomatic ALT and AST elevations, but no jaundice; ALT 378, 670, 830 U/L at 6-8 days, recovery in <7 days; biopsy in 2 showed minimal changes, occasional ballooned cell, scattered foci of inflammation and necrosis and acidophilic bodies).
- Goldenberg DL. Letter: Aspirin hepatotoxicity. Ann Intern Med. 1974;80:773. [PubMed: 4832164](Letter in response to article by Seaman [1974], reporting case of aspirin hepatotoxicity in 16 year old with systemic lupus, given aspirin [2.4 g/day] for 10 days with AST 540 U/L, Alk P 150 U/L, salicylates 11 mg/dL: ALT remaining elevated for further 6 months and rising to >1000 U/L when switched to choline salicylate, resolving within 10 days of stopping).
- Hilton AM, Dymock IW. Letter: Aspirin hepatotoxicity. Ann Intern Med. 1974;81:271–2. [PubMed: 4846112](Letter in response to article by Seaman [1974] suggesting that mild, underlying chronic liver disease is common in lupus and may account for ALT elevations).
- Seaman WE, Ishak KG, Plotz PH. Letter: Aspirin and hepatotoxicity. Ann Intern Med. 1974;80:279. addendum. [PubMed: 4811807](Reply to Hilton [1974], mentioning that re-biopsy of patient with aspirin hepatotoxicity 18 months later showed no chronic liver disease, but rather minimal, nonspecific changes).
- Wolfe JD, Metzger AL, Goldstein RC. Aspirin hepatitis. Ann Intern Med. 1974;80:74–6. [PubMed: 4810352](Young patient with lupus was given aspirin [4.8 g/day for 5 days] and developed symptoms of anorexia and nausea [no jaundice, ALT 168 U/L, Alk P 197], resolving in 12 days: rechallenge caused recurrence in 2 days [ALT 300 U/L] and biopsy showed minimal changes: Case 1).
- Zimmerman HJ. Editorial: Aspirin-induced hepatic injury. Ann Intern Med. 1974;80:103–5. [PubMed: 4810329](Editorial accompanying article by Seaman [1974] makes point of high dose aspirin being a direct hepatotoxin, causing self-limited, rapidly resolving hepatocellular injury; 3-5 g/day yielding serum levels ~20 to 45 mg/dL [2-5 mM], a level rarely reached by other drugs).
- Athreya BH, Moser G, Cecil HS, Myers AR. Aspirin-induced hepatotoxicity in juvenile rheumatoid arthritis. A prospective study. Arthritis Rheum. 1975;18:347–52. [PubMed: 1156454](ALT, AST or Alk P elevations occurred in 22 of 34 children with juvenile rheumatoid arthritis on aspirin, usually mild, correlating poorly with dose and serum levels, some resolving despite continuation of aspirin; no mention of bilirubin or symptoms).
- Bar-Meir S, Papa MZ. Harefuah. 1975;88:241. [Liver damage following salicylate intake] Hebrew. [PubMed: 1132819]
- Garber E, Craig RM, Bahu RM. Letter: Aspirin hepatotoxicity. Ann Intern Med. 1975;82:592–3. [PubMed: 1119782](Case of aspirin liver injury in patient without rheumatologic disorder taking 8 g/day of aspirin for headaches [ALT 111 U/L, Alk P 108 U/L], liver biopsy showing focal necrosis and inflammation, resolving within 7 days of stopping).
- Linnemann CC, Shea L, Partin JC, Schubert WK, Schiff GM. Reye's syndrome: epidemiologic and viral studies, 1963-1974. Am J Epidemiol. 1975;101:517–26. [PubMed: 1155430](Study of 58 children with Reye syndrome: 3 had recurrence; 6 occurred after chickenpox, but most after influenza B; 36% mortality, average symptoms for 7 days, 91% took salicylates, 88% had positive salicylate levels).
- Sillanpää M, Mäkelä AL, Koivikko A. Acute liver failure and encephalopathy (Reye's syndrome?) during salicylate therapy. Acta Paediatr Scand. 1975;64:877–80. [PubMed: 1189911](13 year old with fever and joint aches given salicylates, developed upper respiratory infection followed by confusion and coma [bilirubin 1.4 mg/dL, AST 2500 U/L], with rapid recovery and no recurrence of arthritis; no liver biopsy and unclear whether it was acute salicylate toxicity or Reye syndrome).
- Daum F, Zucker P, Cohen MI. Acute liver failure and encephalopathy (Reye's syndrome?) during salicylate therapy. Acta Paediatr Scand. 1976;65:747. [PubMed: 998231]PubMed Citation (Letter in response to article by Sillanpaa [1975] suggesting that liver biopsy is necessary for diagnosis of Reye syndrome).
- Zucker P, Daum F, Cohen MI. Aspirin hepatitis. Am J Dis Child. 1975;129:1433–4. [PubMed: 1199984](Two children on high dose aspirin [2.4-3.6 g/day] developed abdominal pain and AST elevations [400 and 1290 U/L], resolving with stopping, positive rechallenge in one; review of literature).
- Levy G, Yaffe SJ. Clinical implications of salicylate-induced liver damage. Am J Dis Child. 1975;129:1385–6. [PubMed: 1199979](Comment in response to article by Zucker [1975], suggesting monitoring and dose adjustment when using aspirin).
- Athreya BH. Letter: Aspirin hepatitis. Am J Dis Child. 1976;130:676. [PubMed: 937290](Letter in response to Zucker [1975] mentioning their publication [Athreya 1973]).
- Barone R, Chase PH, Wallace SL. Letter: Salicylate-induced hepatic injury. Arthritis Rheum. 1976;19:964–6. [PubMed: 962975](Elderly woman given aspirin [3 g/day] for fractured ankle developed ALT elevations [20 rising to 402 U/L] after 12 days, resolving in 12 days, with positive rechallenge [salicylate levels ~25 mg/dL]).
- Halla JT. Aspirin, liver, and rheumatic diseases. J Med Assoc State Ala. 1976;46:23–5. [PubMed: 789798]
- Iancu T, Elian E. Ultrastructure changes in aspirin hepatotoxicity. Am J Clin Path. 1976;66:570–5. [PubMed: 961636](Electron microscopy of liver biopsy from child with rheumatic fever with ALT elevations [240 U/L] on aspirin: marked ER dilation, pleomorphic mitochondria with increased density and fat droplets).
- Miller JJ 3rd, Weissman DB. Correlations between transaminase concentrations and serum salicylate concentration in juvenile rheumatoid arthritis. Arthritis Rheum. 1976;19:115–8. [PubMed: 1252262](Among 92 children with juvenile rheumatoid arthritis, 41% had ALT and 59% AST elevations on aspirin; usually mild; poor correlation with serum salicylate levels, dose or duration of therapy; biopsy in one showed minor nonspecific changes).
- Rachelefsky GS, Kar NC, Coulson A, Sarkissian E, Stiehm ER, Paulus HE. Serum enzyme abnormalities in juvenile rheumatoid arthritis. Pediatrics. 1976;58:730–6. [PubMed: 980605](Mild and fluctuating ALT and AST elevations found in 37 children with juvenile rheumatoid arthritis without apparent correlation with aspirin use or levels, but dose unclear and not prospectively designed study or analysis).
- Ricks WB. Letter: Salicylate hepatotoxicity in Reiter's syndrome. Ann Intern Med. 1976;84:52–3. [PubMed: 1244795](18 year old man with Reiter syndrome developed rising AST levels [150 to 1200 U/L] while on aspirin [3.6 g daily], which fell rapidly to normal upon stopping and rose again [~350 U/L] within a few days of restarting; liver biopsy showed minimal inflammation and focal areas of necrosis).
- Schulman P. Letter: Salicylate hepatotoxicity and HL-A-W27. Ann Intern Med. 1976;84:754–5. [PubMed: 937899](Letter in response to Ricks [1976] asking whether HLA-W27 was tested in patient with Reiter syndrome; the author replies "no").
- Saltzman DA, Gall EP, Robinson SF. Aspirin-induced hepatic dysfunction in a patient with adult rheumatoid arthritis. Am J Dig Dis. 1976;21:815–20. [PubMed: 786007](Case report of adult with rheumatoid arthritis who developed ALT elevations during aspirin therapy with two positive rechallenges, latency of 5-7 days and peak ALT ~400 U/L without jaundice; liver biopsies showing cellular unrest; possibly microvesicular fat).
- Seaman WE, Plotz PH. Effect of aspirin on liver tests in patients with RA or SLE and in normal volunteers. Arthritis Rheum. 1976;19:155–60. [PubMed: 1259798](Prospective study of 20 patients with rheumatoid arthritis, 16 with lupus and 3 normal volunteers: ALT elevations occurred in 20%, 44% and 33% after 2 weeks; levels being earlier and higher in lupus patients; no difference in albumin levels; hepatomegaly occurred in some and BUN and creatinine elevations in others).
- Wilson JR. Aspirin hepatotoxicity in adults with rheumatoid arthritis. Ohio State Med J. 1976;72:577–8. [PubMed: 967392](2 adult patients with rheumatoid arthritis had ALT elevations [590 and 229 U/L] with no symptoms after few weeks of high dose aspirin therapy [3.6 g/day], having previously tolerated lower doses without ALT elevations).
- Bernstein BH, Singsen BH, King KK, Hanson V. Aspirin-induced hepatotoxicity and its effect on juvenile rheumatoid arthritis. Am J Dis Child. 1977;131:659–63. [PubMed: 868818](Among 102 children with juvenile rheumatoid arthritis on aspirin, 5% had elevations in ALT [91% when tested serially], usually mild and self-limited, correlating poorly with dose and salicylate levels; excellent example of adaptation).
- Fong WL, Wong PO, Rosenthal HL. Aspirin hepatotoxicity: case report. Drug Intell Clin Pharm. 1977;11:750–1.Not in PubMed
- Gitlin N, Grant J. Raised serum transaminase levels in patients with rheumatic fever treated with salicylates. S Afr Med J. 1977;51:697–8. [PubMed: 877782](11 children with acute rheumatoid fever given aspirin and followed for AST and salicylate levels; 6 had AST elevations of 20-505 U/L [only with salicylate levels >19.2 mg/dL] and 10 had eosinophilia >600/μL; all asymptomatic).
- O'Gorman T, Koff RS. Salicylate hepatitis. Gastroenterology. 1977;72(4 Pt 1):726–8. [PubMed: 838229](2 patients with ALT elevations [250 and 1000 U/L] arising after 4 and 7 days of aspirin therapy [5.2 and 2.4 g/day] for rheumatoid arthritis and lupus; biopsies showed lobular disarray and occasional spotty necrosis; rapid recovery).
- O'Gorman T, Koff RS. Wien Med Wochenschr. 1978;128:434. [Salicylate hepatitis] German. [PubMed: 706392](German translation of same article).
- Sbarbaro JA, Bennett RM. Aspirin hepatotoxicity and disseminated intravascular coagulation. Ann Intern Med. 1977;86:183–5. [PubMed: 835940](17 year old with juvenile rheumatoid arthritis developed ALT elevations [>500 U/L, bilirubin 3.0 mg/dL] on aspirin with positive rechallenge and microangiopathic anemia).
- Babb RR. Analgesic hepatotoxicity. West J Med. 1978;129:164–5. [PMC free article: PMC1238300] [PubMed: 695571](Editorial on aspirin and acetaminophen hepatotoxicity without new information).
- Boss G. Internal Medicine-Epitomes of Progress: Hepatotoxicity caused by acetaminophen or salicylates. West J Med. 1978;129:50–1. [PMC free article: PMC1238232] [PubMed: 18748245](Editorial pointing out that chronic high dose salicylate therapy rather than acute overdose leads to hepatotoxicity).
- Bryant CE. Salicylate-induced hepatotoxicity: a review. J Okla State Med Assoc. 1978;71:284–7. [PubMed: 671139](Review article without new information).
- Jespersen J. Ugeskr Laeger. 1978;140:2247–8. [Salicylate hepatitis in rheumatic fever] Danish. [PubMed: 684951]
- Kanada SA, Kolling WM, Hindin BI. Aspirin hepatotoxicity. Am J Hosp Pharm. 1978;35:330–6. [PubMed: 305202](After 11 days of aspirin [3.6-4.8 g/day], 46 year old man developed elevated AST levels [rising from 15 to 160 to 350 U/L] without rise in bilirubin, and return to normal 8 days after switching to ibuprofen).
- Knudsen FU. Ugeskr Laeger. 1978;140:2233–6. [Acetylsalicylic acid and liver damage] Danish. [PubMed: 684947]
- Mesihović H. Med Arh. 1978;32:67–72. [Hepatotoxicity during treatment of juvenile rheumatoid arthritis and rheumatic diseases using salicylates] Croatian. [PubMed: 309537](Among 199 patients with rheumatic disorders, 6 [3%] had raised ALT [44-485 U/L] or AST [60-400 U/L] compared to 12 of 26 [46%] children with juvenile rheumatoid arthritis [ALT 438-296 U/L], arising 1-22 weeks after starting salicylates).
- Petty BG, Zahka KG, Bernstein MT. Aspirin hepatitis associated with encephalopathy. J Pediatr. 1978;93:881–2. [PubMed: 712501](Child with lupus given aspirin [70 mg/kg/day] developed lethargy after 5 days, followed by nausea and disorientation with ALT 340 U/L, bilirubin 0.8 mg/dL, abnormal EEG, liver biopsy showing mild injury and inflammation; rapid resolution, positive rechallenge).
- Schaller JG. Chronic salicylate administration in juvenile rheumatoid arthritis: aspirin "hepatitis" and its clinical significance. Pediatrics. 1978;62 Suppl:916–25. [PubMed: 724343](Review article on salicylates, including hepatotoxicity and 3 case reports, including one with juvenile rheumatoid arthritis and liver disease independent of aspirin and two patients who demonstrated sensitivity to the hepatotoxicity of aspirin).
- Travers RL, Hughes GR. Salicylate hepatotoxicity in systemic lupus erythematosus: a common occurrence? Br Med J. 1978;2:1532–3. [PMC free article: PMC1608782] [PubMed: 728711](7 patients with systemic lupus or connective tissue disorder on aspirin [1.8-3.6 g daily] had AST elevations of 160-300 U/L, all asymptomatic, none jaundiced, all resolving within 10 days of stopping).
- Ulshen MH, Grand RJ, Crain JD, Gelfand EW. Hepatotoxicity with encephalopathy associated with aspirin therapy in rheumatoid arthritis. J Pediatr. 1978;93:1034–7. [PubMed: 722421](3 children with juvenile rheumatoid arthritis developed encephalopathy after 1-3 weeks of aspirin therapy [100-200 mg/day] with AST 1089-5600, bilirubin 2.2-4.7 and salicylate levels >35 mg/dL, with several recurrences on rechallenge, rapid recoveries; liver biopsy in 2 patients showed minimal changes and no mention of microvesicular fat).
- Bulugahapitiya DT, Hebron B, Beck PR. Salicylate hepatitis with acidosis in an infant. Lancet. 1979;1:1295–6. [PubMed: 87756](7 month old developed acidosis [pH 7.35 and 7.2] and ALT elevations [169 and 543 U/L] after being given aspirin for 5-7 days twice; ammonia elevated but no encephalopathy).
- Jespersen J, Ejstrup L. Ugeskr Laeger. 1979;141:2119–21. [Salicylates and liver involvement. Increased aminotransferases in patients with active rheumatoid arthritis under treatment with salicylates] Danish. [PubMed: 483410]
- Lahaie RG, Blondin C, Huet PM. Salicylate hepatitis in a case of juvenile dermatomyositis. Can Med Assoc J. 1979;120:1399–400. [PMC free article: PMC1819351] [PubMed: 455188](Child with dermatomyositis developed nausea and ALT elevations after 2 days of aspirin [ALT 600 U/L, bilirubin 1.2 mg/dL], with positive rechallenge and biopsy showing nonspecific changes).
- Rochanawutanon M, Bunyaratvej S, Israngkura P. Salicylate induced hepatotoxicity in juvenile rheumatoid arthritis--a case report. J Med Assoc Thai. 1979;62:646–51. [PubMed: 512520](7 year old girl with juvenile rheumatoid arthritis on aspirin and corticosteroids for ~5 months developed fever, rectal bleeding and jaundice, and died after unsuccessful surgery and complications; role of aspirin vs sepsis-shock unclear).
- Carneskog J, Florath-Ahlmen M, Olsson R. Prevalence of liver disease in patients taking salicylates for arthropathy. Hepatogastroenterology. 1980;27:361–4. [PubMed: 7203370](Survey of 110 patients with arthritis treated with long term salicylates: 2 with minor ALT elevations and 9 with Alk P increases, but not clearly related to aspirin).
- Gitlin N. Salicylate hepatotoxicity: the potential role of hypoalbuminemia. J Clin Gastroenterol. 1980;2:281–5. [PubMed: 7451927](Among 17 children with acute rheumatoid fever given salicylates [2.4-3.6 g/day], 9 [53%] developed asymptomatic AST elevations [25-270 U/L] directly related to salicylate levels [>15], but also inversely related to albumin suggesting role of free salicylate levels).
- Mäkelä AL, Lang H, Korpela P. Toxic encephalopathy with hyperammonaemia during high-dose salicylate therapy. Acta Neurol Scand. 1980;61:146–56. [PubMed: 7395459](Two case reports of encephalopathy, hyperammonemia and AST elevations arising during high dose aspirin therapy of rheumatic disease in 8 and 13 year old children, both recovered; possibly Reye syndrome; 13 year old previously reported by Sillanpaa [1975]).
- Mastaglia GL. Salicylate hepatotoxicity in rheumatoid arthritis. Med J Aust. 1980;2:341. [PubMed: 7421712](56 year old woman with rheumatoid arthritis given aspirin [3.9 g/day] developed asymptomatic AST elevations [800 U/L], resolving on stopping).
- Bertino JS Jr, Willis ED, Reed MD, Speck WT. Salicylate hepatitis: a complication of the treatment of Kawasaki's disease. Am J Hosp Pharm. 1981;38:1171–2. [PubMed: 7270563](7 week old child with Kawasaki disease given aspirin [100 mg/kg/day] who developed rising AST levels [~1200 U/L] after 2 weeks, falling to normal with 7 days of stopping and remaining normal during lower dose therapy [30 mg/kg/day]).
- Chesta J, Orellana Alcalde JM, Mehana D. Rev Med Chil. 1981;109:1188–90. [Acetylsalicylic acid-induced hepatotoxicity] Spanish. [PubMed: 7346919]
- Zimmerman HJ. Effects of aspirin and acetaminophen on the liver. Arch Intern Med 1981; 141 (3 Spec No): 333-42. [PubMed: 7469624](Summary of 320 reported patients with juvenile rheumatoid arthritis, rheumatic fever, systemic lupus, rheumatoid arthritis and other rheumatic and nonrheumatic conditions who developed hepatoxicity from aspirin; injury was characterized by mild symptoms, marked ALT and AST elevations with bilirubin elevations in only 3% and modest Alk P increases; some cases were severe with coagulopathy and rarely fatal; may be more common in children and possibly in juvenile rheumatoid arthritis, lupus and acute rheumatic fever than in other conditions; the injury probably represents intrinsic toxicity from aspirin).
- Halpin TJ, Holtzhauer FJ, Campbell RJ, Hall LJ, Correa-Villaseñor A, Lanese R, Rice J, Hurwitz ES. Reye's syndrome and medication use. JAMA. 1982;248:687–91. [PubMed: 7097918](Analysis of medication exposure in 97 cases of Reye syndrome and 156 controls from 1978-80, among 90 medications reported, only aspirin differed between groups: 97% vs 71%).
- von Mühlendahl KE. Dtsch Med Wochenschr. 1982;107:101–3. [Salicylate induced disturbances of liver function (author's transl).] German. [PubMed: 7056156](Five children with juvenile rheumatoid arthritis given aspirin [46-100 mg/kg/day] developed ALT elevations of 185-1020 U/L after 17-25 days of therapy; bilirubin and Alk P were normal; resolved in 5-7 days, minimal or no symptoms).
- Partin JS, Partin JC, Schubert WK, Hammond JG. Serum salicylate concentrations in Reye’s disease: a study of 130 biopsy-proven cases. Lancet 1982; 1 (Jan 23): 191-4. [PubMed: 6119559](Serum salicylate levels averaged 15 mg/dL in cases of Reye syndrome but averaged <2 mg/dL in controls; levels did not correlate well with severity of injury; no mention of what proportion of cases had detectable levels).
- Schmidt RE. Dtsch Med Wochenschr. 1982;107:398. [Hepatotoxicity of acetylsalicylic acid] German. [PubMed: 7060471]
- Waldman RJ, Hall WN, McGee H, Van Amburg G. Aspirin as a risk factor in Reye’s syndrome. JAMA. 1982;247:3089–94. [PubMed: 7077803](Case control study of 25 children with Reye syndrome and 46 controls found aspirin taken in 96% vs 74%).
- Benson GD. Hepatotoxicity following the therapeutic use of antipyretic analgesics. Am J Med. 1983;75:85–93. [PubMed: 6359871](Review of hepatotoxicity of aspirin and acetaminophen when used in therapeutic doses recommends use of acetaminophen in patients with preexisting liver disease).
- Hamdan JA, Ahmad MS, Sa'di AR. Salicylate hepatotoxicity in rheumatic fever. Ann Trop Paediatr. 1983;3:89–91. [PubMed: 6197014](Two children with acute rheumatic fever developed symptoms and AST elevations [1650 and 1325 U/L] with normal bilirubin after 12-13 days of aspirin therapy [100 mg/kg/day], resolving with lower doses).
- Starko KM, Mullick FG. Hepatic and cerebral pathology findings in children with fatal salicylate intoxication: further evidence for a causal relation between salicylate and Reye's syndrome. Lancet. 1983;1:326–9. [PubMed: 6185805](Study of 13 children with salicylate intoxication from AFIP files, showing microvesicular fat in liver [usually mild and zone 1] and brains often showed cerebral edema).
- Bhabha FS, Kshirsagar NA, Pohujani S, Dastur P, Joshi MU, Kandoth P, Satoskar RS. Effect of aspirin on renal and hepatic function in children suffering from juvenile rheumatoid arthritis and rheumatic fever. Indian J Pediatr. 1984;51:317–21. [PubMed: 6511049](24 children with juvenile rheumatoid arthritis or rheumatic fever studied prospectively during aspirin therapy [100 mg/kg/day] for 3 weeks; decline in creatinine clearance was common and 3 had asymptomatic ALT elevations [79, 146 and 300 U/L] returning to normal rapidly with stopping).
- Partin JS, Daugherty CC, McAdams AJ, Partin JC, Schubert WK. A comparison of liver ultrastructure in salicylate intoxication and Reye's syndrome. Hepatology. 1984;4:687–90. [PubMed: 6745858](Liver biopsies from two children with aspirin hepatotoxicity showed minimal changes by electron microscopy with none of the mitochondrial enlargement or marked microvesicular fat seen in Reye syndrome despite similar ALT values).
- Young RSK, Torretti D, Williams R, Hendriksen D, Woods M. Reye's syndrome associated with long-term aspirin therapy. JAMA. 1984;251:754–6. [PubMed: 6694278](Two cases and review of literature on Reye syndrome in children on long term aspirin for rheumatologic disorders; 3 and 1 year olds on 80 and 150 mg/kg/day of aspirin for 3 months developed fever and then vomiting and coma with AST 1532 and 3460 U/L, bilirubin 0.7 and 1.0 mg/dL and high ammonia, lactate and protime, both recovered but one left with spastic quadriparesis).
- Hamdan JA, Manasra K, Ahmed M. Salicylate-induced hepatitis in rheumatic fever. Am J Dis Child. 1985;139:453–5. [PubMed: 3984967](Retrospective study of 34 Saudi children with rheumatic fever given salicylates; 5 had ALT elevations [216-1650 U/L], all with salicylate levels >25 mg/dL, rapid resolution, no jaundice but some with symptoms; managed with lower doses).
- Hansen JR, McCray PB, Bale JF Jr, Corbett AJ, Flanders DJ. Reye syndrome associated with aspirin therapy for systemic lupus erythematosus. Pediatrics. 1985;76:202–5. [PubMed: 4022693](14 year old on aspirin for 2 weeks developed fever, malaise and then stupor with AST 2130 U/L, bilirubin 0.8 mg/dL, high protime and ammonia levels with nontoxic salicylate levels; recovered slowly and redeveloped signs of lupus with stopping aspirin; biopsy showed mild microvesicular fat).
- Everson GW, Krenzelok EP. Chronic salicylism in a patient with juvenile rheumatoid arthritis. Clin Pharm. 1986;5:334–41. [PubMed: 3709082](Case report of child with juvenile rheumatoid arthritis treated with aspirin [150 mg/day] developing lethargy, fever and rash progressing to disorientation: salicylate 44.2 mg/dL, ALT 1136 U/L, ammonia 186 μmol/L, bilirubin 2.1 rising to 10.8 mg/dL, protime 29 seconds; recovery in 2 weeks).
- Jakubowska D, Grzebieniak-Kopieczna E. Wiad Lek. 1986;39:1278–83. [Salicylate hepatotoxicity] Polish. [PubMed: 3564467]
- Peters LJ, Wiener GJ, Gilliam J, Van Noord G, Geisinger KR, Roach ES. Reye's syndrome in adults: a case report and review of the literature. Arch Intern Med. 1986;146:2401–3. [PubMed: 3778077](61 year old with febrile illness, took aspirin and 4 days later developed nausea, then confusion and admission for coma 2 days later, ALT 864 U/L, bilirubin and INR normal, liver biopsy showed microvesicular fat, rapid recovery; reviewed 11 cases of Reye syndrome in adults in literature: ages 18-51, receiving aspirin after upper respiratory illness, 3 died).
- Cersosimo RJ, Matthews SJ. Hepatotoxicity associated with choline magnesium trisalicylate: case report and review of salicylate-induced hepatotoxicity. Drug Intell Clin Pharm. 1987;21:621–5. [PubMed: 3301251](Patient with lupus given trisalicylate [1.5 g/day] and in 3 days had rising ALT [711 U/L] and Alk P [376 U/L] but normal bilirubin, resolving rapidly with stopping: review of literature).
- Grigor RR, Spitz PW, Furst DE. Salicylate toxicity in elderly patients with rheumatoid arthritis. J Rheumatol. 1987;14:60–6. [PubMed: 3572936](Analysis of ARAMIS system on 544 patients on salicylates alone in 3 age groups; elderly reported fewer side effects but used lower doses; liver toxicity not mentioned).
- Heubi JE, Partin JC, Partin JS, Schubert WK. Reye's syndrome: current concepts. Hepatology. 1987;7:155–64. [PubMed: 3542776](Extensive review of the history, epidemiology, pathogenesis, etiology, role of salicylates, similarity to inborn errors of metabolism, and animal models of Reye syndrome).
- Hurwitz ES, Barrett MJ, Gunn WJ, Pinsky P, Schonberger LB, Drage JS, et al. Public Health Service Study of Reye's syndrome and medications: report of the main study. JAMA. 1987;257:1905–11. [PubMed: 3820509](Final analysis of prospective, case-control study of 27 patients with Reye syndrome presenting between 1985-6 and 140 controls showing salicylate use in 96% of cases vs 32% controls, odds ratio=40, doses of aspirin were not excessive, 90% had history of a preceding respiratory tract infection).
- Freeland GR, Northington RS, Hedrich DA, Walker BR. Hepatic safety of two analgesics used over the counter: ibuprofen and aspirin. Clin Pharmacol Ther. 1988;43:473–9. [PubMed: 3365912](Analysis of database on 1468 patients with rheumatoid arthritis, including 439 given aspirin [2.6-3.9 g/day] found AST elevations in 4% to 22%; 10 patients had ALT >3 times ULN [2%], ibuprofen had lowest rate, oxaprozin intermediate).
- Porter JDH, Robinson PH, Glasgow JFT, Banks JH., Hall SM. Trends in the incidence of Reye's syndrome and the use of aspirin. Arch Dis Child. 1990;65:826–9. [PMC free article: PMC1792465] [PubMed: 2400216](British Surveillance System data showed a marked decrease in use of aspirin in children with fever after public warning in 1986 and that the frequency of Reye syndrome subsequently declined from peak of 79 cases in 1983-4 to 19 in 1988-9).
- De Leeuw P, Lefebvre C, Tomasi JP, Rahier J, Geubel A. Gastroenterol Clin Biol. 1992;16:359–61. [Severe hepatitis with encephalopathy induced by acetylsalicylic acid in a case of lupus erythematosus disseminatus] French. [PubMed: 1397856](62 year old woman with systemic lupus on long term therapy with aspirin, had dose increased to 3.5 g/day and 10 days later developed weakness and confusion with ALT 34 times ULN, Alk P 2.9 times ULN, bilirubin 15.8 mg/dL, high ammonia; biopsy did not show microvesicular fat; resolved on low dose prednisone).
- Nadkarni MM, Peller CA, Retig J. Eosinophilic hepatitis after ingestion of choline magnerium trisalicylate. Am J Gastroenterol. 1992;87:151–3. [PubMed: 1728115](66 year old woman with osteoarthritis took 3 days of trisalicylate and developed jaundice 3 days later with ALT 774 U/L, Alk P 542 U/L, bilirubin 14.3 mg/dL; 32% eosinophils, mild encephalopathy, but ultimate recovery; had a history of penicillin allergy).
- Peraf F, Kovacs T, Jothy S. Syndrome de Reye de l'adulte. Gastroenterol Clin Biol. 1992;16:483–5. [Reye's syndrome in adults] French. [PubMed: 1526408](24 year old with history of fever followed by somnolence had taken aspirin in unknown amounts and developed coma with ammonia 138 μmol/L, ALT 64 times ULN, Alk P <1 times ULN and bilirubin 1.5 mg/dL, with eventual rapid recovery).
- Singh H, Chugh JC, Shembesh AH, Ben-Musa AA, Mehta HC. Hepatotoxicity of high dose salicylate therapy in acute rheumatic fever. Ann Trop Paediatr. 1992;12:37–40. [PubMed: 1376585]
- Chan ED. Reye's syndrome in a young adult. Mil Med. 1993;158:65–8. [PubMed: 8437745](26 year old woman with a 4 day history of cough and fever developed confusion, [bilirubin 0.8 mg/dL, ALT 683 U/L, ammonia 192 μmol/L, elevated lactic acid] and died within 2 weeks, with autopsy showing microvesicular fatty liver).
- López-Morante AJ, Sáez-Royuela F, Díez-Sánchez V, Martín-Lorente JL, Yuguero L, Ojeda C. Aspirin-induced cholestatic hepatitis. J Clin Gastroenterol. 1993;16:270–2. [PubMed: 8505510](72 year old man with stroke developed cholestatic jaundice after 1 month of low dose aspirin [bilirubin 11.5 mg/dL, ALT 211 U/L, Alk P 646 U/L] with normal ERCP, no other drug exposure, resolving in 10 weeks off of aspirin).
- Mallet EC, Gestas P, Vic P, Arnaud JP. Arch Fr Pediatr. 1993;50:272–3. [Fulminant hepatitis with encephalopathy in acute articular rheumatism treated with acetylsalicylic acid] French. [PubMed: 8338425](5 year old child with acute arthritis placed on aspirin [100 mg/kg/day] and developed confusion, nausea and vomiting 4 days later with subsequent fatal course [ALT 1230 U/L, pH 7.26, high ammonia and lactate but normal bilirubin], liver biopsy showing microvesicular fat).
- Rubie H, Guillot S, Netter JC, Le Tallec C, Voigt JJ, Claeyssens S, Olives JP, et al. Arch Pediatr. 1994;1:573–7. [Acute hepatopathy compatible with Reye's syndrome in 3 children treated by chemotherapy] French. [PubMed: 7994349](3 children developed Reye-like syndrome 1-2 months after course of chemotherapy of leukemia with acute encephalopathy, ALT elevations [2.5 to 4.5 times ULN], no jaundice, but microvesicular fat on liver biopsy; no history of aspirin use but one had prodromal febrile illness).
- Sizykh TP, Efimova NIu. Probl Tuberk. 1994;(4):57–60. [Liver function in patients with aspirin-induced bronchial asthma] Russian. [PubMed: 7984621]
- Hardie RM, Newton LH, Bruce JC, Glasgow JF, Mowat AP, Stephenson JB, Hall SM. The changing clinical pattern of Reye's syndrome: 1982-1990. Arch Dis Child. 1996;74:400–5. [PMC free article: PMC1511546] [PubMed: 8669954](Application of a scoring system for 445 cases of Reye syndrome showed that cases were fewer as well as milder after 1986).
- Belay ED, Bresee JS, Holman RC, Khan AS, Shahriari A, Schonberger LB. Reye's syndrome in the United States from 1981 through 1997. N Engl J Med. 1999;340:1377–82. [PubMed: 10228187](National Surveillance Data from 1980-97 of Reye syndrome in children <18 years showed a sharp decline with recognition of role of aspirin [1980], Surgeon General's advisory [1982] and aspirin labeling [1986], from peak of 555 cases in 1980 to <37 in 1987-93, to <2 in 1994-7: the case fatality rate was 31%).
- Singh H, Chugh JC. Hepatotoxicity of salicylate therapy in acute rheumatic fever. Indian Pediatr. 1999;36:611. Erratum in: Indian Pediatr 1999; 36: 904. [PubMed: 10736599](Letter regarding review article on aspirin for rheumatic fever pointing out their results [Singh 1992]).
- Caksen H, Guler E, Alper M, Ustunbas HB. A fatal case of Reye syndrome after varicella and ingestion of aspirin. J Dermatol. 2001;28:286–7. [PubMed: 11436370](4 year old with varicella treated with aspirin developed stupor one week later [ALT 150 U/L, ammonia 853 μmol/L, protime 27 seconds, and acidosis], and died within 3 days: microvesicular fat on autopsy).
- Chen TC, Ng KF, Jeng LB, Yeh TS, Chen CM. Aspirin-related hepatotoxicity in a child after liver transplant. Dig Dis Sci. 2001;46:486–8. [PubMed: 11318519](4 year old child with biliary atresia, developed CMV infection after liver transplantation and was treated with aspirin for fever [Tapal 100 mg three times daily], developing ALT flare to 459 U/L, resolving within 20 days of stopping aspirin).
- McGovern MC, Glasgow JF, Stewart MC. Lesson of the week: Reye's syndrome and aspirin: lest we forget. BMJ. 2001;322:1591–2. [PMC free article: PMC1120628] [PubMed: 11431304](Two cases of Reye syndrome in children who took aspirin during febrile illnesses [AST 1113 and 1034 U/L, ammonia 181 and 108 μmol/L and protime 15.1 and 41 seconds], both recovered).
- da Silveira EBV, Young K, Rodriguez M, Ameen N. Reye's syndrome in a 17-year-old male: is this disease really disappearing? Dig Dis Sci. 2002;47:1959–61. [PubMed: 12353836](17 year old boy took aspirin for febrile illness and presented with confusion 6 days later with ALT 1728 U/L, normal bilirubin; ultrasound showed fatty liver and biopsy showed microvesicular fat; recovered in 5 days).
- Bhutta AT, Savell VH, Schexnayder SM. Reye's syndrome: down but not out. South Med J. 2003;96:43–5. [PubMed: 12602712](Case report of 3 year old boy given aspirin for fever presenting a few days later with encephalopathy and acute liver failure [bilirubin 1.1 mg/dL, AST 1060 U/L, Alk P 272 U/L, acidosis], liver biopsy showed microvesicular fat; patient had brain herniation and died: first case report from US in many years).
- Chow EL, Cherry JD, Harrison R, McDiarmid SV, Bhuta S. Reassessing Reye Syndrome. Arch Pediatr Adolesc Med. 2003;157:1241–2. [PubMed: 14662583](10 year old girl given aspirin for febrile illness developed confusion 2 days later [ALT 598 U/L, INR 1.9, ammonia 250 μmol/dL], liver biopsy showed microvesicular fat; ultimately suffered brain death).
- Karademir S, Oğuz D, Senocak F, Ocal B, Karakurt C, Cabuk F. Tolmetin and salicylate therapy in acute rheumatic fever: Comparison of clinical efficacy and side-effects. Pediatr Int. 2003;45:676–9. [PubMed: 14651540](72 children with rheumatic fever were given tolmetin [n=20] or aspirin [n=52: 75-100 mg/kg/day] for 4-6 weeks; efficacy similar but side effects were more common with aspirin, ALT elevations [100-1000 U/L, but no jaundice] in 31% with aspirin vs 0% with tolmetin).
- Lacroix I, Lapeyre-Mestre M, Bagheri H, Pathak A, Montastruc JL. Club de Reflexion des cabinets de Groupe de Gastro-Enterologie (CREGG).; General Practitioner Networks. Nonsteroidal anti-inflammatory drug-induced liver injury: a case-control study in primary care. Fundam Clin Pharmacol. 2004;18:201–6. [PubMed: 15066135](Case controlled study of cases of NSAID hepatotoxicity versus controls seen in a medical community care group in France, found 22 cases of NSAID induced hepatotoxicity among a total of 88 cases; including 7/88 attributable to aspirin, but 10/178 controls also taking aspirin; odds ratio=1.42; no clinical data given on cases).
- van Bever HP, Quek SC, Lim T. Aspirin, Reye syndrome, Kawasaki disease, and allergies; a reconsideration of the links. Arch Dis Child. 2004;89:1178. [PMC free article: PMC1719775] [PubMed: 15557065](Hypothesis linking rise in allergic diseases among children and decrease in use of aspirin: "It may not be too bold a postulate that this increase in allergic diseases might be due [at least in part] to the decreased use of ASA").
- Wei C-M, Chen H-L, Lee P-I, Chen C-M, Ma C-Y, Hwu W-L. Reye's syndrome developing in an infant on treatment of Kawasaki syndrome. J Paediatr Child Health. 2005;41:303–4. [PubMed: 15953335](1 year old boy with Kawasaki syndrome was treated with IVIG and high dose aspirin and developed lethargy 2-3 days later with ALT 376 U/L, high ammonia, lactic acidosis and coagulopathy; given glucose and mannitol and recovered within a few days: liver biopsy showed microvesicular fat; no recurrence 2 years later, but no mention of restarting aspirin).
- Ioi H, Kawashima H, Nishimata S, Watanabe Y, Yamanaka G, Kashiwagi Y, Yamada N, et al. A case of Reye syndrome with rotavirus infection accompanied with high cytokines. J Infect. 2006;52:e124–8. [PubMed: 16226809](2 year old with diarrhea from rotavirus infection followed by coma and seizures with ALT 4136 U/L and hypoglycemia but no acidosis or jaundice biopsy showed fatty liver; ultimately recovered, did not take aspirin and possibility of metabolic disorder not fully investigated).
- Glasgow JF. Reye's syndrome: the case for a causal link with aspirin. Drug Saf. 2006;29:1111–21. [PubMed: 17147458](Systematic review of evidence linking aspirin ingestion to Reye syndrome; 6 case-control studies demonstrated significant increase in risk and 2 long-term prospective surveillance studies with 450 confirmed cases showed aspirin use in 59% of cases and 26% of controls, mortality rate 53%; marked decrease in numbers since 1996; cases without aspirin exposure may have inherited metabolic disease resembling Reye syndrome).
- Schrör K. Aspirin and Reye syndrome: a review of the evidence. Paediatr Drugs. 2007;9:195–204. [PubMed: 17523700](Review article questioning the cause-and-effect relationship between aspirin intake and Reye syndrome and suggesting that the increase prevalence of asthma in children may relate to decrease in aspirin or increase in acetaminophen use).
- Fitzgerald DA. Aspirin and Reye syndrome. Paediatr Drugs. 2007;9:205–6. [PubMed: 17523701](Editorial on controversy surrounding aspirin and Reye syndrome).
- Jiménez-Caballero PE, Montes-Gonzalo MC, Velázquez-Pérez JM. Rev Neurol. 2008;47:571–4. [Reye's syndrome. Description of a case focused on the patient's epileptic seizures] Spanish. [PubMed: 19048536]
- Gosalakkal JA, Kamoji V. Reye syndrome and Reye-like syndrome. Pediatr Neurol. 2008;39:198–200. [PubMed: 18725066](14 year old girl developed fever followed by vomiting and confusion having taken 3 doses of aspirin [325 mg each] 3 days earlier [ALT 80 U/L, ammonia 120 μmol/L, glucose 81 mg/dL, pH 7.34] and died 5 days later).
- Pugliese A, Beltramo T, Torre D. Reye's and Reye's-like syndromes. Cell Biochem Funct. 2008;26:741–6. [PubMed: 18711704](Review of Reye syndrome and its differential diagnosis which includes congenital metabolic disorders of urea cycle, and fatty acid and glucose metabolism).
- Ghosh A, Pradhan S, Swami R. K C SR, Talwar OP. Reye syndrome: a case report with review of literature. JNMA J Nepal Med Assoc. 2008;47:34–7. [PubMed: 18552890](3 year old Nepalese girl developed fever followed by vomiting and loss of consciousness [ALT 1270 U/L] and died 2 days later, autopsy showing hepatic microvesicular steatosis; no history of aspirin ingestion).
- Beutler AI, Chesnut GT, Mattingly JC, Jamieson B. FPIN's Clinical Inquiries. Aspirin use in children for fever or viral syndromes. Am Fam Physician. 2009;80:1472. [PubMed: 20000310](Addresses the question of the age at which aspirin is safe to use for viral syndromes or fever in children; rare cases have been reported in adults, and most recommendations are to not use aspirin below the age of 19 years).
- Lemberg A, Fernández MA, Coll C, Rosello DO, Romay S, Perazzo JC, Filinger EJ. Reyes's syndrome, encephalopathy, hyperammonemia and acetyl salicylic acid ingestion in a city hospital of Buenos Aires, Argentina. Curr Drug Saf. 2009;4:17–21. [PubMed: 19149521](12 cases of Reye syndrome presented to a referral hospital in Buenos Aires between 1985 and 2000; ages 2 to 10 years, 6 boys, all with fever followed by vomiting and encephalopathy; all had taken aspirin [average ALT 302 U/L, glucose 35 mg/dL], 5 died).
- Cağ M, Saouli AC, Audet M, Wolf P, Cinqualbre J. Reye syndrome and liver transplantation. Turk J Pediatr. 2010;52:662–4. [PubMed: 21428204](6 month old boy with fever and cough received a single dose of aspirin and rapidly developed seizures and coma with hypoglycemia [glucose 17 mg/dL, ALT 75 rising to 11,270 U/L], recovering with emergency auxiliary partial liver transplant; explant showing microvesicular steatosis and in follow up, the native liver recovered and so that immunosuppression could be withdrawn, but he had severe epilepsy and delayed psychomotor development in long term follow up).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to aspirin).
- Ozsoylu S. Did the patient have Reye syndrome? Turk J Pediatr. 2011;53:356. [PubMed: 21980824](Letter in response to Cağ [2010] suggesting that the patient had an inherited mitochondrial condition and Reye-like syndrome).
- Whitten R, Milner DA Jr, Yeh MM, Kamiza S, Molyneux ME, Taylor TE. Liver pathology in Malawian children with fatal encephalopathy. Hum Pathol. 2011;42(9):1230–9. [PMC free article: PMC3137725] [PubMed: 21396681](Autopsy results on 87 Malawian children who died of fatal encephalopathy suspected to be due to cerebral malaria identified 9 with marked hepatic microvesicular steatosis with minimal necrosis suggestive of Reye syndrome, 6 of whom had a recent history of aspirin ingestion).
- Link A, Kaplan BT, Böhm M. Dtsch Med Wochenschr. 2012;137(38):1853–6. [21-year-old woman with Reye's syndrome after influenza] German. [PubMed: 22971971](21 year old woman developed coma shortly after recovering from influenza for which she took aspirin [bilirubin not given, ALT 548 U/L, INR 1.58, ammonia 358 μg/dL], with progressive neurologic deterioration and death).
- Raza A, Vierling J, Hussain KB. Genetics of drug-induced hepatotoxicity toxicity in Gilbert's syndrome. Am J Gastroenterol. 2013;108:1936–7. [PubMed: 24300875](55 year old man with elevated liver tests [bilirubin 1.4 mg/dL, ALT 219 U/L, Alk P 72 U/L] while taking an analgesic with aspirin [1.5 g/day] and acetaminophen [1.5 g/day], had Gilbert syndrome and was homozygous for the A(TA)7TAA promoter mutation).
- Selves A, Ruiz S, Crognier L, Conil JM, Bonneville F, Georges B, Dupuy M, et al. Ann Fr Anesth Reanim. 2013;32:814–6. [Aspirin and its danger: Reye syndrome in young adult] French. [PubMed: 24161294](19 year old man developed coma after receiving aspirin for viral pericarditis and died of cerebral edema, liver tests were minimally abnormal [bilirubin 1.2 mg/dL, ALT normal, AST 1.5 times ULN], serum ammonia and liver biopsy not available).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, Presentation and Outcomes in Patients with Drug-Induced Liver Injury in the General Population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to allopurinol).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to aspirin).
- Laster J, Satoskar R. Aspirin-induced acute liver injury. ACG Case Rep J. 2014;2:48–9. [PMC free article: PMC4435341] [PubMed: 26157904](41 year old woman with pericarditis developed abdominal pain and marked elevations in serum aminotransferase levels 2 days after starting high doses of aspirin [bilirubin 0.8 mg/dL, ALT 5684 U/L, Alk P 84 U/L, INR 13.8], resolving rapidly upon stopping).
- Shirota T, Ikegami T, Sugiyama S, Kubota K, Shimizu A, Ohno Y, Mita A, et al. Successful living donor liver transplantation for acute liver failure after acetylsalicylic acid overdose. Clin J Gastroenterol. 2015;8:97–102. [PubMed: 25711165](20 year old Japanese woman took an overdose of aspirin [66 grams] and developed liver and renal failure, pancreatitis and progressive coma, and was successfully treated with emergency liver donor left lobe transplantation).
- Cao Z, Liu S, Niu J, Wei B, Xu J. Severe hepatoxicity caused by aspirin overdose: a case report. Front Med. 2015;9:388–91. [PubMed: 26085469](61 year old man took an overdose of aspirin [4 g] and developed gastrointestinal bleeding, fever, pneumonia, cholecystitis, pancreatitis and multiorgan failure [bilirubin peak 18.1 mg/dL, ALT 7790 U/L, Alk P not given], resolving over the following month).
- Ahrens-Nicklas RC, Edmondson AC, Ficicioglu C. An 8-year-old girl with abdominal pain and mental status changes. Pediatr Emerg Care. 2015;31:459–62. [PubMed: 26035505](8 year old girl was treated with aspirin for suspected rheumatic fever and developed vomiting, diarrhea and progressive somnolence [bilirubin 0.7 mg/dL, ALT 2834 U/L, Alk P not given, INR 1.67, ammonia 161 μ mol/L, glucose 57 mg/dL], recovering in several days with conservative management).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to aspirin).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Aspirin and Reye syndrome: a review of the evidence.[Paediatr Drugs. 2007]Review Aspirin and Reye syndrome: a review of the evidence.Schrör K. Paediatr Drugs. 2007; 9(3):195-204.
- National patterns of aspirin use and Reye syndrome reporting, United States, 1980 to 1985.[Pediatrics. 1987]National patterns of aspirin use and Reye syndrome reporting, United States, 1980 to 1985.Arrowsmith JB, Kennedy DL, Kuritsky JN, Faich GA. Pediatrics. 1987 Jun; 79(6):858-63.
- The anti-inflammatory, analgesic and antipyretic activities of non-narcotic analgesic drug mixtures in rats.[Arch Int Pharmacodyn Ther. 1981]The anti-inflammatory, analgesic and antipyretic activities of non-narcotic analgesic drug mixtures in rats.Seegers AJ, Jager LP, Zandberg P, van Noordwijk J. Arch Int Pharmacodyn Ther. 1981 Jun; 251(2):237-54.
- Review Acetylsalicylic acid and acetaminophen.[Dent Clin North Am. 1994]Review Acetylsalicylic acid and acetaminophen.Kacso G, Terézhalmy GT. Dent Clin North Am. 1994 Oct; 38(4):633-44.
- Reye Syndrome.[StatPearls. 2024]Reye Syndrome.Chapman J, Arnold JK. StatPearls. 2024 Jan
- Aspirin - LiverToxAspirin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...