NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Diflunisal is a salicylic acid derivative that is used in the therapy of chronic arthritis and mild to moderate acute pain. Diflunisal has been linked mild, transient elevations in serum aminotransferase levels during therapy as well as to rare instances of idiosyncratic drug induced liver disease.
Background
Diflunisal (dye floo' ni sal) is a difluorophenol derivative of salicylic acid that has antiinflammatory, analgesic and antipyretic actions similar to aspirin. Diflunisal is metabolized by the liver, but not to salicylate. Diflunisal is considered a nonsteroidal antiinflammatory agent (NSAID) and is believed to act by inhibition of tissue cyclo-oxygenases (Cox-1 and Cox-2), which leads to a decrease in synthesis of proinflammatory prostaglandins, important mediators in inflammatory and pain pathways. Diflunisal was approved for use in the United States in 1982 and current indications are for chronic arthritis due to osteoarthritis or rheumatoid arthritis and for mild-to-moderate pain. Diflunisal has been shown to stabilize transthyretin variants which are involved in the pathogenesis of amyloidosis, which has led to the off-label use of diflunisal in familial amyloidosis. Diflunisal is available as tablets of 500 mg in generic forms and formerly under the brand name of Dolobid. Diflunisal is available by prescription only. The recommended regimen in adults is an initial dose of 1000 mg, followed by 500 to 1500 mg daily in two to three divided doses based upon response and tolerance. Diflunisal, like most NSAIDs, is generally well tolerated, but side effects can include intestinal upset, nausea, heartburn, headache, somnolence, dizziness, peripheral edema and hypersensitivity reactions.
Hepatotoxicity
Diflunisal therapy is reported to be associated with a low rate of asymptomatic and transient serum aminotransferase elevations, which may resolve even with drug continuation. Marked aminotransferase elevations (>3 fold elevated) occur rarely. Clinically apparent liver injury with jaundice from diflunisal is uncommon; only case reports have been published. The clinical and histologic features of diflunisal hepatotoxicity, however, are distinct and resemble an immunoallergic hepatitis, which is quite different from the liver injury that occurs with aspirin or other salicylates (Case 1). The latency to onset ranges from 1 to 4 weeks and the pattern of enzyme elevations is typically cholestatic, but can also be mixed. Most patients have immunoallergic manifestations such as rash, fever and arthralgias; eosinophilia or atypical lymphocytosis are also common. A history of aspirin allergy has not been reported among cases with allergic reactions to diflunisal. Diflunisal is not a commonly used drug and is not mentioned in large case series on drug induced liver injury or acute liver failure.
Likelihood score: C (probable cause of clinically apparent liver injury).
Mechanism of Liver Injury
The mechanism of diflunisal hepatotoxicity is likely hypersensitivity and is different from that of acetylsalicylic acid (aspirin).
Outcome and Management
Severity ranges from asymptomatic elevations in serum aminotransferase levels, to symptomatic cholestatic hepatitis with or without jaundice. It is not clear if there is cross sensitivity to liver injury between diflunisal and other salicylates.
Drug Class: Salicylates, Nonsteroidal Antiinflammatory Drugs
CASE REPORT
Case 1. Cholestatic jaundice arising after a few days of diflunisal therapy.(1)
A 65 year old woman with venous stasis ulcers was given diflunisal (750 mg daily) for leg pain while being treated with intravenous penicillin for suspected cellulitis. When therapy was started, serum enzymes and bilirubin levels were normal (Table). Diflunisal was stopped after 3 days because of gastrointestinal intolerance. Five days later, blood tests showed marked elevations of alkaline phosphatase with mild hyperbilirubinemia. There was eosinophilia but no rash, lymphadenopathy or further fever. She had had a cholecystectomy in the past, but no history of liver disease or risk factors for viral hepatitis were mentioned. Ultrasound of the abdomen and endoscopic retrograde cholangiopancreatography were unrevealing. A liver biopsy showed portal inflammation with eosinophils and lobular histiocytic granulomas. Over the next weeks, laboratory test abnormalities fell toward normal despite continuation of penicillin and her medications for depression (clorazepate and viloxazine). Four months later all blood test results were normal.
Key Points
| Medication: | Diflunisal (750 mg daily for 3 days) |
|---|---|
| Pattern: | Cholestatic (R=0.3) |
| Severity: | 3+ (jaundice and prolongation of hospitalization) |
| Latency: | 3 days to symptoms, 8 days to detection of jaundice |
| Recovery: | 2-4 months |
| Other medications: | Penicillin (iv), clorazepate, viloxazine |
Laboratory Values
| Time After Starting | Time After Stopping | AST (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| 1 day | 0 | 13 | 80 | 0.3 | eosinophils 2% |
| 8 days | 5 days | 60 | 1216 | 2.6 | eosinophils 12% |
| 13 days | 10 days | 50 | 1474 | 1 | Liver biopsy |
| 30 days | 27 days | 16 | 672 | 0.6 | eosinophils 34% |
| 2 months | 2 months | 19 | 193 | 0.3 | eosinophils 23% |
| 5 months | 5 months | 18 | 70 | 0.2 | eosinophils 3% |
| Normal Values | Not given | ||||
Comment
Despite absence of some clinical information, this is a convincing case of cholestatic jaundice caused by diflunisal. As in other cases, the latency was quite short and there was at least some evidence for an immunoallergic reaction (eosinophilia). Granulomas found on liver biopsy do not indicate “granulomatous hepatitis”, but are rather further evidence of a generalized immunoallergic reaction. The rapid clinical recovery (1 month), but delayed fall of alkaline phosphatase to the normal range (between 2 and 5 months) is typical of cholestatic drug induced liver injury.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Diflunisal – Generic
DRUG CLASS
Antiinflammatory Agents
Product labeling at DailyMed, National Library of Medicine, NIH.
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
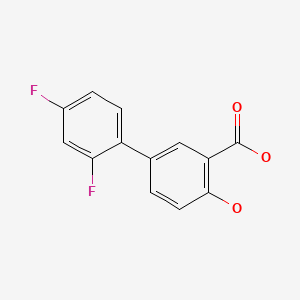
| Diflunisal | 22494-42-4 | C13-H8-F2-O3 |
|
CITED REFERENCE
- 1.
- Geoffroy P, Carteret E, Salagnac V, Kalis B, Zeitoun P. Therapie. 1987;42:253. [Hepatitis caused by diflunisal (Dolobis). Apropos of a case] French. [PubMed: 3617008]
ANNOTATED BIBLIOGRAPHY
References updated: 03 January 2018
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. Chapter 19: The NSAIDS. In Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott Williams & Williams, 1999, pp. 517-41.(Review of hepatotoxicity of NSAIDs published in 1999).
- Lewis JH, Stine JG. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 369-402.(Expert review of liver injury caused by NSAIDs mentions that diflunisal is a difluorophenol derivative of salicylate and can cause cholestatic injury but that there have been few published reports).
- Grosser T, Smyth E, FitzGerald GA. Anti-inflammatory, antipyretic, and analgesic agents; pharmacotherapy of gout. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 959-1004.(Textbook of pharmacology and therapeutics).
- Warren JS. Diflunisal-induced cholestatic jaundice. Br Med J. 1978;2:736–7. [PMC free article: PMC1607620] [PubMed: 698699](64 year old man who was previously treated with multiple NSAIDs without relief was placed on diflunisal [while also receiving indomethacin] and developed itching 5 days later [bilirubin 3.4 mg/dL, AST 120 U/L, Alk P 780 U/L] without eosinophilia, rash or fever, and resolving within 2 months of stopping).
- Brogden RN, Heel RC, Pakes GE, Speight TM, Avery GS. Diflunisal: a review of its pharmacological properties and therapeutic use in pain and musculoskeletal strains and sprains and pain in osteoarthritis. Drugs. 1980;19:84–106. [PubMed: 6988202](Review of pharmacology, pharmacokinetics, clinical efficacy and safety of diflunisal; "no consistent alteration in tests of renal or liver function have been reported").
- Umbenhauer ER. Diflunisal in the treatment of the pain of osteoarthritis. Summary of clinical studies. Pharmacotherapy. 1983;3:55S–60S. [PubMed: 6344040](Review of results of 5 controlled studies of diflunisal vs aspirin or ibuprofen or placebo in 1218 patients; side effects were more frequent with aspirin and ibuprofen than diflunisal but discontinuations for AST elevations occurred in 0.9% of diflunisal- vs 1% of aspirin- and none of ibuprofen-treated patients).
- Geoffroy P, Carteret E, Salagnac V, Kalis B, Zeitoun P. Therapie. 1987;42:253. [Hepatitis caused by diflunisal (Dolobis). Apropos of a case] French. [PubMed: 3617008](65 year old woman developed mild cholestatic jaundice 5 days after a 3 day course of diflunisal [bilirubin 2.6 mg/dL, AST 60 U/L and Alk P 1216 U/L, eosinophilia], resolving in several months: Case #1).
- Cook DJ, Achong MR, Murphy FR. Three cases of diflunisal hypersensitivity. CMAJ. 1988;138:1029–30. [PMC free article: PMC1267889] [PubMed: 2967101](Three patients with onset of fever, rash and eosinophilia [23-31%] or atypical lymphocytosis after 2-4 weeks of diflunisal therapy responding to oral and topical steroids; with bilirubin levels of normal, 0.9 and 4.9 mg/dL, ALT normal, 500 and 810 U/L, Alk P 185, 545 and 349 U/L).
- Zimmerman HJ. Update of hepatotoxicity due to classes of drugs in common clinical use: non-steroid drugs, anti-inflammatory drugs, antibiotics, antihypertensives, and cardiac and psychotropic agents. Semin Liver Dis. 1990;10:322–8. [PubMed: 2281340](Extensive review article on liver injury due to NSAIDs; diflunisal said to cause cholestatic injury rarely).
- Muller AF, Toghill PJ, Smith P. ‘Relapse’ of chronic active hepatitis--not always what it seems. Postgrad Med J. 1996;72:431–2. [PMC free article: PMC2398514] [PubMed: 8935606](Patient with typical autoimmune hepatitis treated with excellent response with steroids and then switched to azathioprine alone for 6 years; developed jaundice 1 month after starting diflunisal [bilirubin 2.9 mg/dL, ALT 668 U/L, Alk P 262 U/L], resolving upon stopping).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](Survey of all cases of drug induced liver injury with fatal outcome from Swedish Adverse Drug Reporting system from 1966-2002; among 103 cases, none were attributed to diflunisal).
- Rostom A, Goldkind L, Laine L. Nonsteroidal anti-inflammatory drugs and hepatic toxicity: a systematic review of randomized controlled trials in arthritis patients. Clin Gastroenterol Hepatol. 2005;3:489–98. [PubMed: 15880319](Review of randomized clinical trials of NSAIDS for frequency of adverse events; ALT elevations above 3 fold ULN occurred in 0.43% of ibuprofen, 0.43% naproxen, 0.42% celecoxib, 1.8% rofecoxib, 3.55% diclofenac and 0.29% of placebo recipients; review deals largely with commonly used NSAIDs and does not mention diflunisal).
- Lapeyre-Mestre M, de Castro AM, Bareille MP, et al. Non-steroidal anti-inflammatory drug-related hepatic damage in France and Spain: analysis from national spontaneous reporting systems. Fundam Clin Pharmacol. 2006;20:391–5. [PubMed: 16867024](Analysis of reports of liver injury from NSAIDs from France and Spain from 1982-2001; diflunisal is not listed in tables of adverse event reports from NSAIDs).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, NSAIDs were implicated as a sole agent in 8 cases [4 diclofenac, 2 celecoxib, 1 meloxicam and 1 oxaprozin] and as one of several agents in 3 cases [1 diclofenac, 1 celecoxib, 1 ibuprofen]; diflunisal is not mentioned).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which 7 were due to NSAIDs, including 4 attributed to bromfenac, 2 to diclofenac and 1 to etodolac, but none to diflunisal).
- Gulmez SE, Larrey D, Pageaux GP, Lignot S, Lassalle R, Jové J, Gatta A, et al. Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case-population SALT study. Drug Saf. 2013;36:135–44. [PMC free article: PMC3568201] [PubMed: 23325533](Analysis of cases of idiopathic acute liver failure undergoing liver transplantation in 52 centers in Europe between 2005 and 2007, found 40 had been exposed to an NSAID, but none to diflunisal).
- Lapeyre-Mestre M, Grolleau S, Montastruc JL., Adsociation Française des Centres Régionaux de Pharmacovigilance (CRPV). Adverse drug reactions associated with the use of NSAIDs: a case/noncase analysis of spontaneous reports from the French pharmacovigilance database 2002-2006. Fundam Clin Pharmacol. 2013;27:223–30. [PubMed: 21929527](Analysis of 42,389 spontaneous serious adverse event reports to the French Pharmacovigilance database on 8 NSAIDs between 2002 and 2006; liver adverse events were most frequent with nimesulide [0.15 per million daily doses] compared to diclofenac [0.09], ketoprofen [0.09] piroxicam [0.06], naproxen [0.04], meloxicam [0.03], and tenoxicam [0.03]; diflunisal is not discussed).
- Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, Heneghan MA, et al. Diflunisal Trial Consortium. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310:2658–67. [PMC free article: PMC4139164] [PubMed: 24368466](Among 130 patients with familial amyloid polyneuropathy treated with diflunisal [250 mg] or placebo twice daily for 2 years, disease progression was less with diflunisal therapy and the overall rates of adverse events were similar with no mention of ALT elevations or hepatotoxicity and no liver related serious adverse events or deaths).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, Presentation and Outcomes in Patients with Drug-Induced Liver Injury in the General Population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, 6% of which were attributed to NSAIDS, but none to diflunisal).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury from Latin American countries published between 1996 and 2012 identified 176 cases; while NSAIDs were the most frequent class of drugs implicated (n=62: 32%], no case was attributed to diflunisal).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 20 cases [2%] were attributed to NSAIDs, but none to diflunisal).
- Sekijima Y, Tojo K, Morita H, Koyama J, Ikeda S. Safety and efficacy of long-term diflunisal administration in hereditary transthyretin (ATTR) amyloidosis. Amyloid. 2015;22:79–83. [PubMed: 26017328](Among 40 Japanese patients with familial amyloidosis treated with diflunisal [500 mg daily] for up to 10 years, 3 patients stopped therapy because of side effects [thrombocytopenia and renal dysfunction which improved upon stopping]; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Rofecoxib.[LiverTox: Clinical and Researc...]Review Rofecoxib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Direct determination of closely overlapping drug mixtures of diflunisal and salicylic acid in serum by means of derivative matrix isopotential synchronous fluorescence spectrometry.[Anal Chim Acta. 2007]Direct determination of closely overlapping drug mixtures of diflunisal and salicylic acid in serum by means of derivative matrix isopotential synchronous fluorescence spectrometry.Murillo Pulgarín JA, Alañón Molina A, Fernández López P, Sánchez-Ferrer Robles I. Anal Chim Acta. 2007 Jan 30; 583(1):55-62. Epub 2006 Oct 10.
- Treatment of mild to moderate pain of acute soft tissue injury: diflunisal vs acetaminophen with codeine.[J Fam Pract. 1986]Treatment of mild to moderate pain of acute soft tissue injury: diflunisal vs acetaminophen with codeine.Muncie HL Jr, King DE, DeForge B. J Fam Pract. 1986 Aug; 23(2):125-7.
- Diflunisal pharmacodynamics in experimental arthritis in rats.[J Rheumatol. 1988]Diflunisal pharmacodynamics in experimental arthritis in rats.Walker JS, Kasmerski L. J Rheumatol. 1988 Nov; 15(11):1643-7.
- Review Diflunisal: a review of its pharmacological properties and therapeutic use in pain and musculoskeletal strains and sprains and pain in osteoarthritis.[Drugs. 1980]Review Diflunisal: a review of its pharmacological properties and therapeutic use in pain and musculoskeletal strains and sprains and pain in osteoarthritis.Brogden RN, Heel RC, Pakes GE, Speight TM, Avery GS. Drugs. 1980 Feb; 19(2):84-106.
- Diflunisal - LiverToxDiflunisal - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...