NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Vorapaxar is an inhibitor of platelet aggregation that is used to decrease the risk of further cardiovascular thrombotic events in patients with a history of myocardial infarction or peripheral vascular disease. Vorapaxar therapy is associated with a low rate of serum aminotransferase elevations, but has not been linked to instances of clinically apparent acute liver injury.
Background
Vorapaxar (vor" a pax' ar) is an inhibitor of the protease-activated receptor 1 (PAR-1) that is found on platelets and is activated by thrombin resulting in platelet aggregation and formation of a vascular clot. The aggregation of platelets also plays an important role in the growth of atheromatous plaques, which can lead to coronary, cerebral and peripheral arterial occlusions. In large clinical trials, vorapaxar therapy has been shown to decrease the frequency of recurrence of myocardial infarction and cardiovascular events in patients at high risk of complications of atherosclerosis. Vorapaxar was approved for use in the United States in 2014 and current indications are for reduction of atherosclerotic events (myocardial infarction, vascular death) in patients with a history of myocardial infarction or peripheral vascular disease. Vorapaxar is contraindicated in patients with a history of stroke or transient ischemic attacks because of an increased risk of intracerebral bleeding. Vorapaxar is available in 2.08 mg tablets under the brand name Zontivity. The usual dose is one tablet (2.08) mg daily. Side effects are not common, but can include headache, dizziness, fatigue, gastrointestinal upset, nausea, arthralgia and rash. Uncommon, but potentially severe adverse reactions including bleeding and particularly intracerebral bleeding in patients with a history of stroke.
Hepatotoxicity
Vorapaxar is associated with a low rate of serum enzyme elevations during therapy that was similar to the rate that occurred with placebo or comparator therapies. In a large controlled trial in over 10,000 patients monitored over a 2 year period, ALT elevations above 5 times the upper limit of normal occurred in 1% of vorapaxar vs 1.4% of placebo patients. In pooled analyses of laboratory studies from more than 39,000 patients receiving vorapaxar or placebo, GGT was the only liver test that was abnormal in a higher proportion of patients receiving vorapaxar (3.8%) than placebo (3.3%), and there were no reports of liver related serious adverse events or clinically apparent liver injury. Thus, liver injury from vorapaxar must be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which vorapaxar might cause liver injury is not known. It is metabolized in the liver, largely via CYP 3A4 and is susceptible to drug-drug interactions, particularly with potent inhibitors or induces of CYP 3A activity.
Outcome and Management
There is little evidence that cangrelor can cause liver injury or has any cross sensitivity to other antiplatelet agents.
Drug Class: Antithrombotic Agents, Antiplatelet Agents
Other Drugs in the Subclass, Antiplatelet Agents: Aspirin, Cangrelor, Clopidogrel, Dipyridamole, Prasugrel, Ticagrelor, Ticlopidine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Vorapaxar – Generic, Zontivity®
DRUG CLASS
Antithrombotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Vorapaxar | 618385-01-6 | C29-H33-F-N2-O4 |
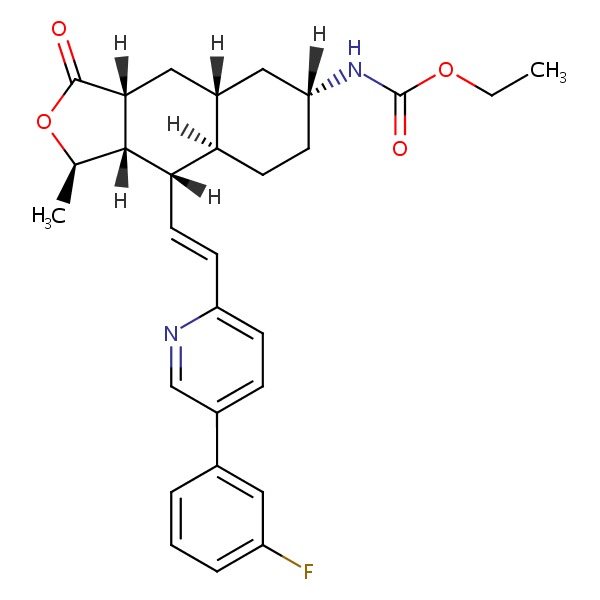 |
ANNOTATED BIBLIOGRAPHY
References updated: 11 April 2017
- Zimmerman HJ. Platelet aggregation inhibitors. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 641-3.(Textbook of hepatotoxicity published in 1999 discusses ticlopidine, but not vorapaxar).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs: antihypertensives. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 528.(Review of hepatotoxicity of antiplatelet drugs; does not discuss vorapaxar).
- Weitz JI. Blood coagulation and anticoagulant, fibrinolytic, and antiplatelet drugs. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 849-76.(Textbook of pharmacology and therapeutics).
- Becker RC, Moliterno DJ, Jennings LK, Pieper KS, Pei J, Niederman A, Ziada KM, et al.; TRA-PCI Investigators. Safety and tolerability of SCH 530348 in patients undergoing non-urgent percutaneous coronary intervention: a randomised, double-blind, placebo-controlled phase II study. Lancet 2009; 373 (9667): 919-28. [PubMed: 19286091](Among 1060 patients undergoing percutaneous coronary intervention given a loading dose followed by oral vorapaxar for 60 days or placebo, clinically significant bleeding occurred at similar rates in all groups; other adverse events were not discussed).
- Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, et al.; TRA 2P–TIMI 50 Steering Committee and Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012; 366: 1404-13. [PubMed: 22443427](Among 26,449 patients with a history of myocardial infarction, stroke or peripheral artery disease treated with vorapaxar [2.5 mg daily] or placebo for a median of 30 months, cardiovascular endpoints were less in the vorapaxar treated group, but moderate-to-severe bleeding episodes [4.2% vs 2.5%] and intracranial hemorrhage [1% vs 0.5%] were more common; other adverse events were not discussed).
- Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, et al.; TRACER Investigators. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 2012; 366: 20-33. [PubMed: 22077816](Among 12,944 patients with acute coronary syndromes treated with vorapaxar or placebo for 1-2 years, intracranial hemorrhage was more frequent with vorapaxar [1.1% vs 0.2%], while other adverse events were similar including any ALT elevations [5.4% vs 6.4%] and those above 5 times ULN [1.0 % vs 1.4%]).
- Vorapaxar (Zontivity) for prevention of thrombotic cardiovascular events. Med Lett Drugs Ther 2014; 56 (1451): 85-6. [PubMed: 25211301](Concise review of the mechanism of action, clinical efficacy, safety and costs of vorapaxar shortly after its approval for use in the US; mentions side effects of bleeding, but does not mention ALT elevations or hepatotoxicity).
- Magnani G, Bonaca MP, Braunwald E, Dalby AJ, Fox KA, Murphy SA, Nicolau JC, et al. Efficacy and safety of vorapaxar as approved for clinical use in the United States. J Am Heart Assoc 2015; 4 (3): e001505. [PMC free article: PMC4392433] [PubMed: 25792124](Secondary analysis of TRA-2P TIMI 50 [Morrow 2012] limiting cohorts to patients without a history of stroke found reduced rates of cardiovascular endpoints at 3 years with vorapaxar therapy compared to placebo [7.9% vs 9.5%]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to vorapaxar or other antiplatelet drugs).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Vorapaxar for reduction of thrombotic cardiovascular events in myocardial infarction and peripheral artery disease.[Am J Health Syst Pharm. 2015]Review Vorapaxar for reduction of thrombotic cardiovascular events in myocardial infarction and peripheral artery disease.Arif SA, D'Souza J, Gil M, Gim S. Am J Health Syst Pharm. 2015 Oct 1; 72(19):1615-22.
- Review Vorapaxar: A Protease-Activated Receptor Antagonist for the Prevention of Thrombotic Events.[Cardiol Rev. 2015]Review Vorapaxar: A Protease-Activated Receptor Antagonist for the Prevention of Thrombotic Events.Lam S, Tran T. Cardiol Rev. 2015 Sep-Oct; 23(5):261-7.
- Vorapaxar in the treatment of cardiovascular diseases.[Future Cardiol. 2020]Vorapaxar in the treatment of cardiovascular diseases.Tantry US, Bliden KP, Chaudhary R, Novakovic M, Rout A, Gurbel PA. Future Cardiol. 2020 Sep; 16(5):373-384. Epub 2020 Apr 20.
- Review Adjunctive therapies to reduce thrombotic events in patients with a history of myocardial infarction: role of vorapaxar.[Drug Des Devel Ther. 2015]Review Adjunctive therapies to reduce thrombotic events in patients with a history of myocardial infarction: role of vorapaxar.Farag M, Patel H, Gorog DA. Drug Des Devel Ther. 2015; 9:3801-9. Epub 2015 Jul 22.
- Review Improving Outcomes in Cardiovascular Diseases: A Review on Vorapaxar.[Cardiol Rev. 2022]Review Improving Outcomes in Cardiovascular Diseases: A Review on Vorapaxar.Chaudhary R, Mohananey A, Sharma SP, Singh S, Singh A, Kondur A. Cardiol Rev. 2022 Sep-Oct 01; 30(5):241-246. Epub 2021 Mar 19.
- Vorapaxar - LiverToxVorapaxar - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...