NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Donahue KE, Gartlehner G, Schulman ER, et al. Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Jul. (Comparative Effectiveness Review, No. 211.)

Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update [Internet].
Show detailsOverview of Content
This appendix contains the results of primary network meta-analyses (NWMA) based on studies with low or medium risk of bias but not shown in our main report because they rendered mostly inconclusive findings with wide confidence intervals. Specifically, these analyses evaluated Disease Activity Score (DAS) remission (Appendix Figure H-2). The network diagram for this outcome are presented in Appendix Figure H-1.
Additionally, we present full forest plots presenting our NWMA across all comparisons (and not within each comparison section) for every outcome of interest discussed in the main report: American College of Rheumatology response defined by 50 percent improvement (ACR50), radiographic joint damage, overall discontinuation, and discontinuation due to adverse events. These appear in Appendix Figure H-4, Appendix Figure H-6, Appendix Figure H-8, and Appendix Figure H-9, respectively, and network diagrams for these outcomes appear in Appendix Figure H-3, Appendix Figure H-5, and Appendix Figure H-7 (for both discontinuation outcomes), respectively.
Appendix Table H-1 lists the specific studies with low or medium risk of bias and reporting on outcomes of interest for our NWMA. These outcomes include DAS remission (n=10), ACR50 (n=11), radiographic joint damage (n=10), overall discontinuation (n=10), and discontinuation due to adverse events (n=12).
Appendix Table H-1Studies included in any KQ1 or KQ3 primary network meta-analyses
| Treatment Comparison | Study Name | DAS Remissiona | Overall D/Ca | D/C due to AEsa | ACR50a | Radiographic joint damagea |
|---|---|---|---|---|---|---|
| ABA+MTX vs. MTX | AGREE, 2009,31 2011,129, 130 2015131 | X | X | X | X | X |
| ABA+MTX vs. ABA vs. MTX | AVERT, 20157 | X | X | X | X | |
| ADA+MTX vs. ADA vs. MTX | PREMIER, 2006,15 2008,103 2010,115 2012,116 2013,117 2014,118 2015119 | X | X | X | ||
| CZP+MTX vs. MTX | C-EARLY, 201738, 39 | X | X | X | X | X |
| ETN vs. MTX | Enbrel ERA, 2000,14 2002,110 2005,164 2006111 | X | X | X | X | |
| ETN+MTX vs. MTX | COMET, 2008,12 2009,154 2010,108, 109 2012;155 2014,156 | X | X | X | X | X |
| IFX+MTX vs. MTX | ASPIRE, 2004,17 2006,107 2009,106 2017157 | X | X | X | X | X |
| IFX+MTX vs. Methyl-PNL+MTX vs. MTX | Durez et al., 200718 | X | X | X | X | X |
| IFX+MTX vs. MTX | Quinn et al., 200541 | X | X | X | ||
| SSZ+MTX vs. SSZ vs. MTX | Dougados et al., 1999;21 Maillefert et al., 2003104 | X | X | X | ||
| SSZ+MTX vs. SSZ vs. MTX | Haagsma et al., 199723 | X | X | |||
| TCZ+MTX vs. TCZ vs. MTX | FUNCTION, 2016,32 2017134 | X | X | X | X | X |
| TCZ+MTX vs. TCZ vs. MTX | U-Act-Early, 201633 | X | X | X | X | X |
ABA = abatacept; ACR50 = American College of Rheumatology 50% improvement; ADA = adalimumab; AE = adverse event; AGREE = Abatacept trial to Gauge Remission and joint damage progression in methotrexate-naïve patients with Early Erosive rheumatoid arthritis; ASPIRE = Active-controlled Study of Patients receiving Infliximab for the treatment of Rheumatoid arthritis of Early onset trial; AVERT = Assessing Very Early Rheumatoid arthritis Treatment trial; C-EARLY = trial whose acronym not described; C-OPERA = Certolizumab-Optimal Prevention of joint damage for Early RA trial; COMET = Combination of Methotrexate and Etanercept in Active Early Rheumatoid Arthritis trial; CZP = certolizumab pegol; D/C = discontinuation; DAS = Disease Activity Score; Enbrel ERA = Enbrel Early RA trial; ETN = etanercept; FUNCTION = trial whose acronym not described; IFX = infliximab; Methyl-PNL = methylprednisolone; MTX = methotrexate; NA = not applicable; NWMA = network meta-analysis; PREMIER = trial whose acronym not described; RA = rheumatoid arthritis; ROB = risk of bias; SSZ = sulfasalazine; TCZ = tocilizumab; U-Act-Early = Trial whose acronym not described; vs. = versus
- a
All data used in NWMA were measured at the 1-year follow-up timepoint.
Network Diagrams and Forest Plots
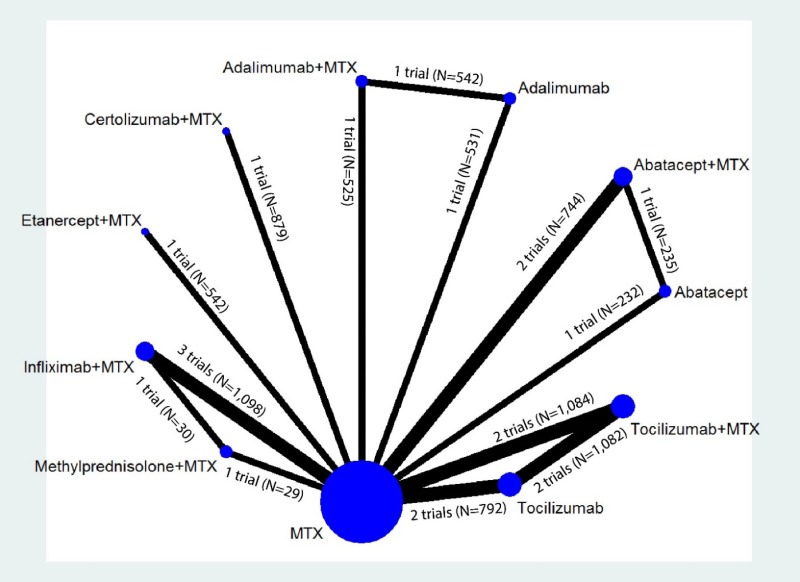
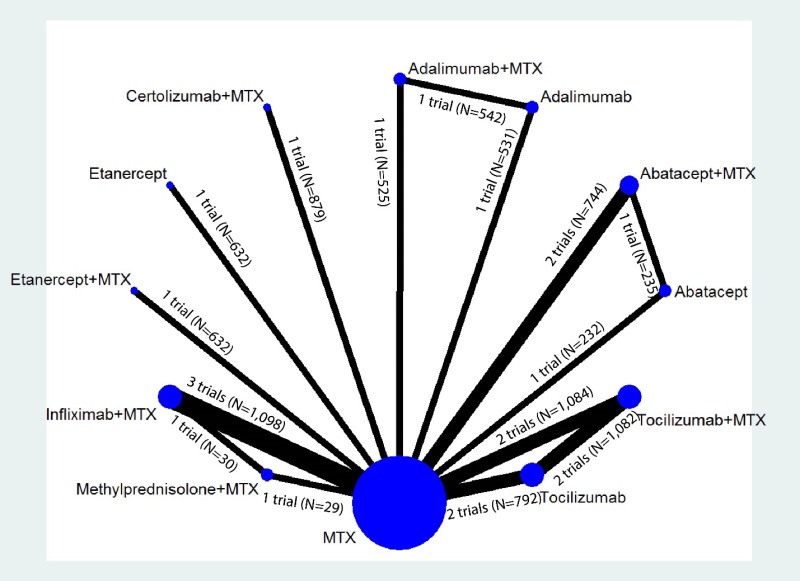
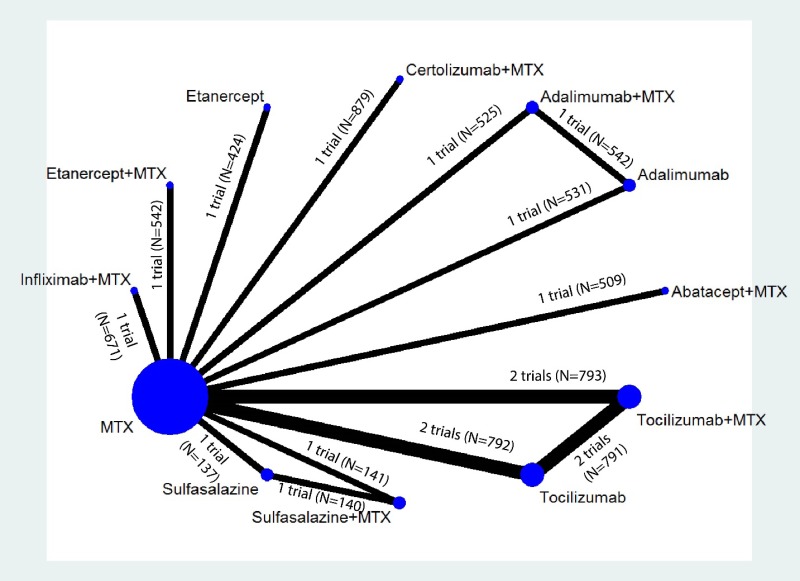
Appendix Figure H-1Network diagram for network meta-analysis: Remission according to Disease Activity Score
MTX = methotrexate; N = number of patients
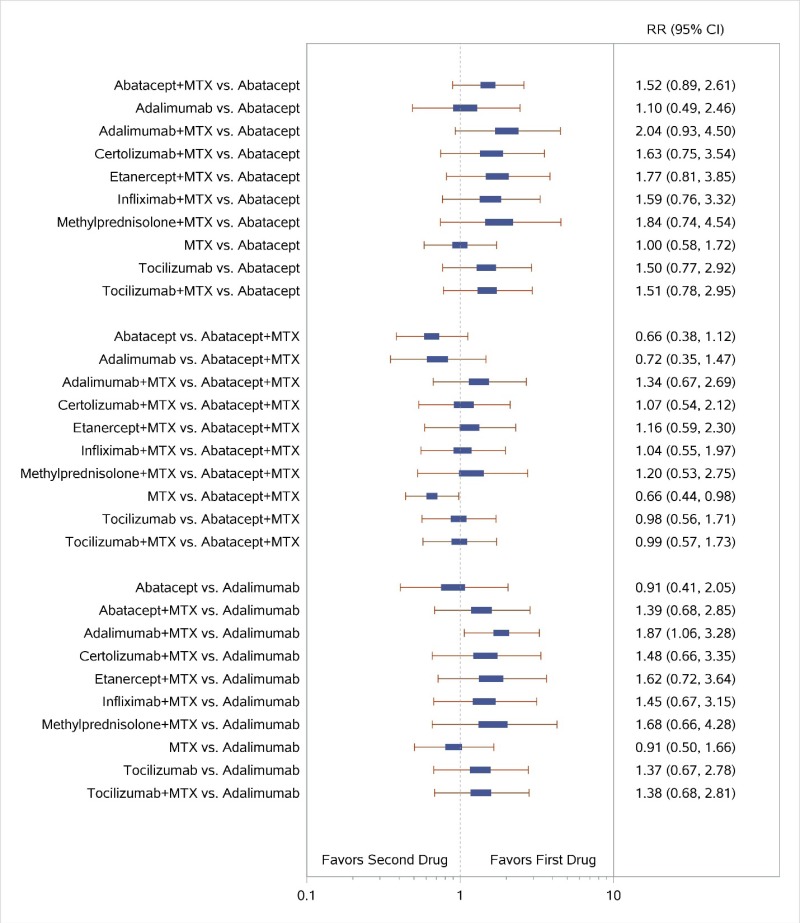
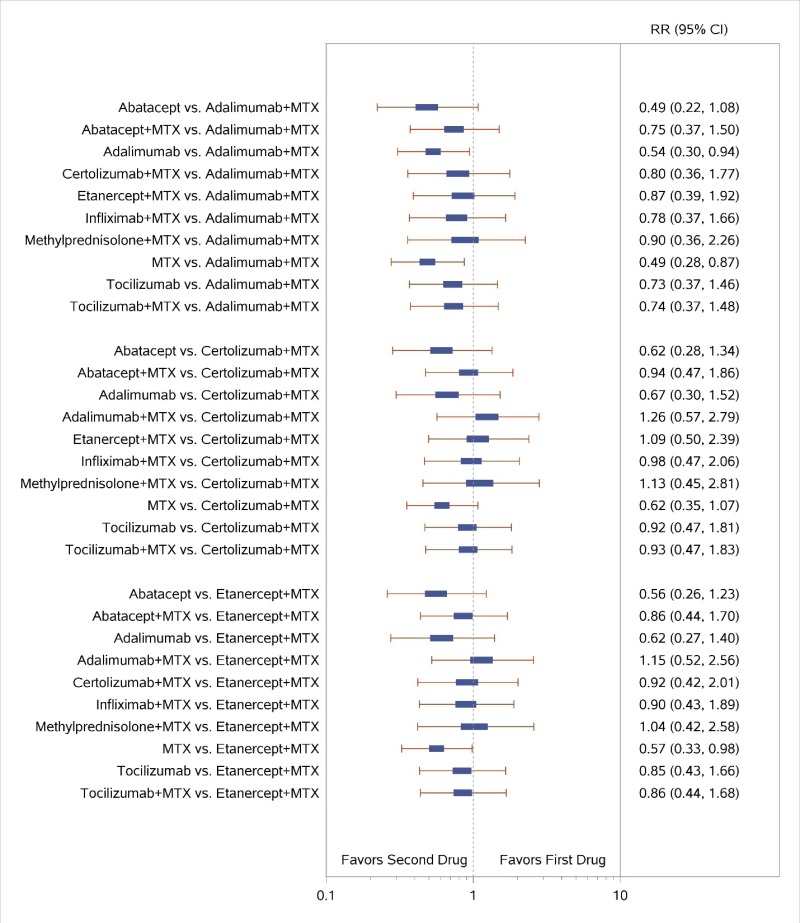
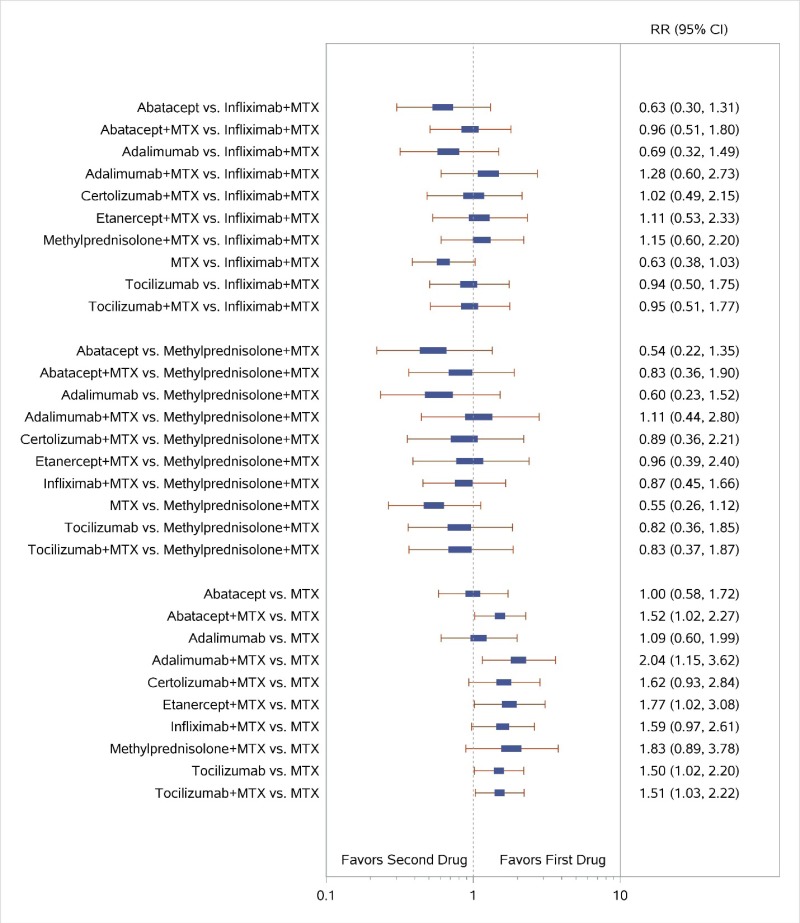
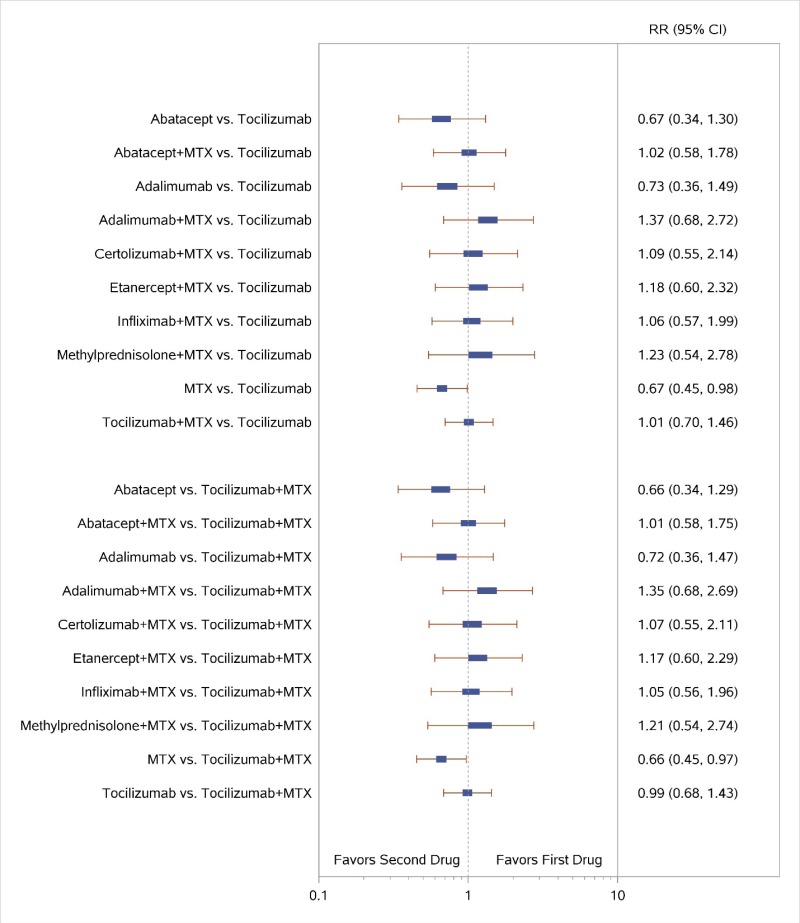
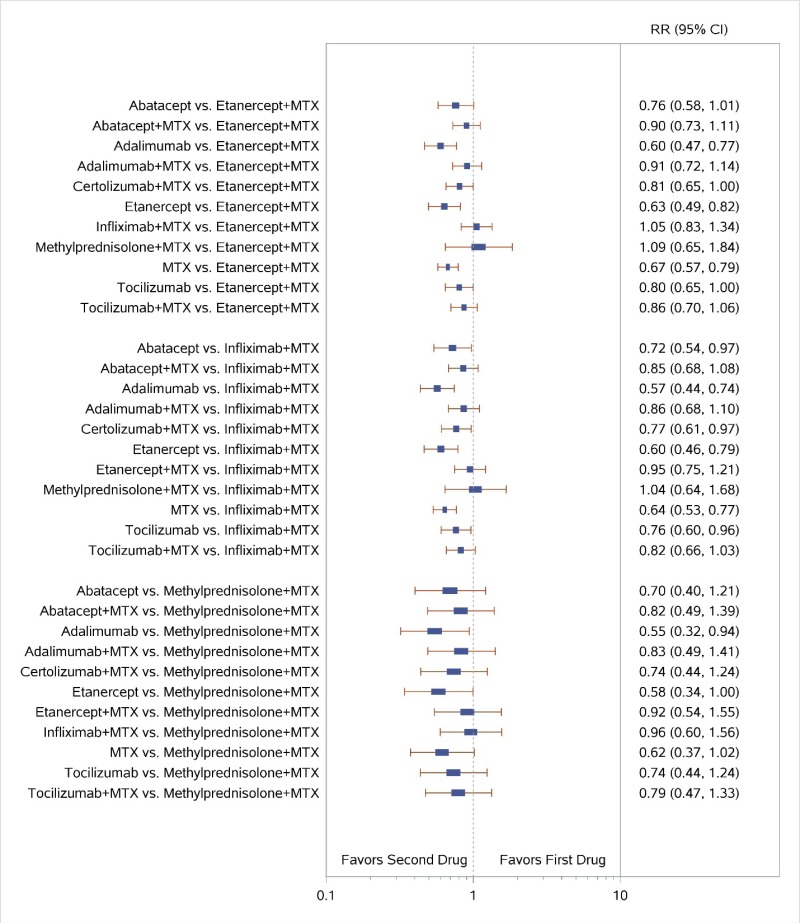
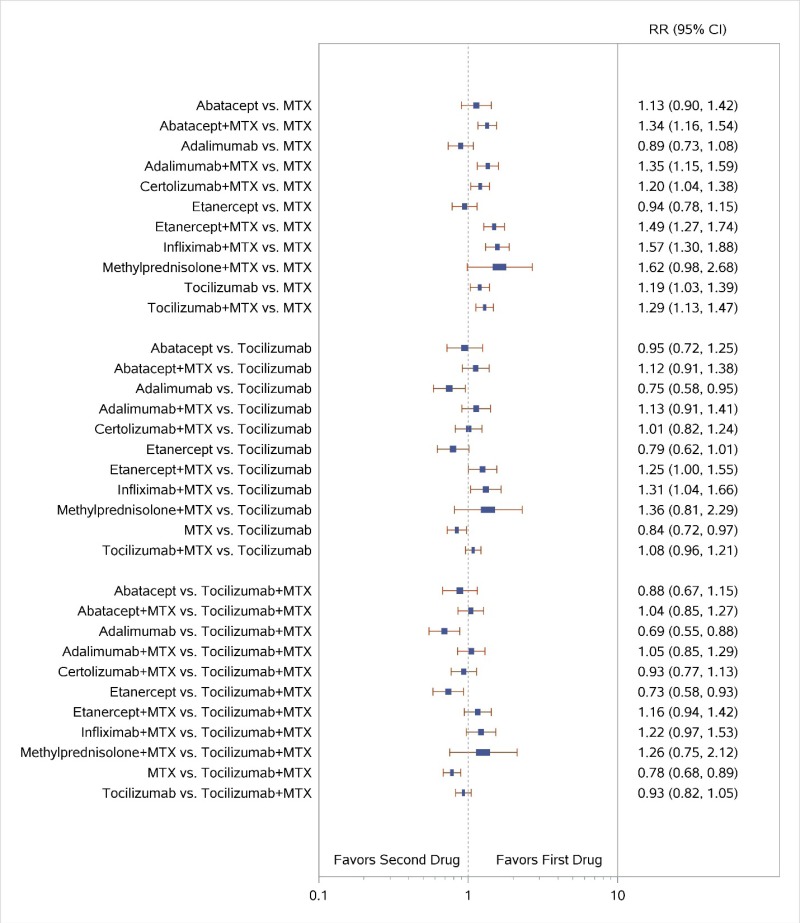
Appendix Figure H-2Forest plots for network meta-analysis: Remission according to Disease Activity Score
MTX = methotrexate; RR = relative risk; vs. = versus; 95% CI = 95% confidence interval
Appendix Figure H-3Network diagram for network meta-analysis: ACR50 response
ACR50 = American College of Rheumatology 50% improvement; MTX = methotrexate; N = number of patients
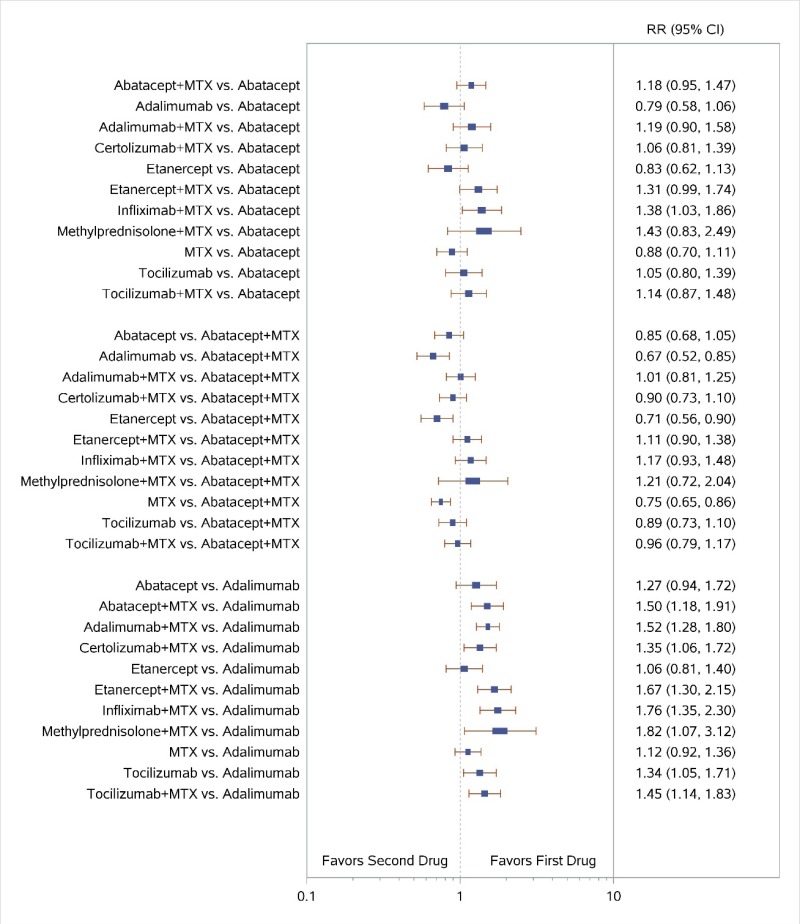
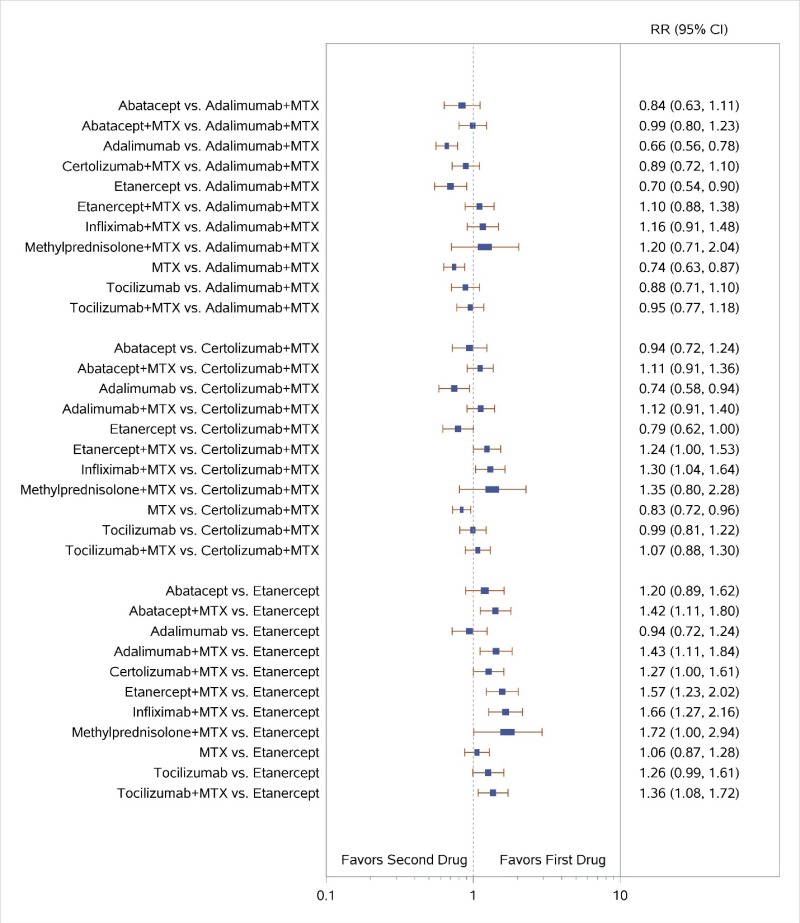
Appendix Figure H-4Forest plots for network meta-analysis of ACR50 response
ACR50 = American College of Rheumatology 50% improvement; MTX = methotrexate; RR = relative risk; vs. = versus; 95% CI = 95% confidence interval
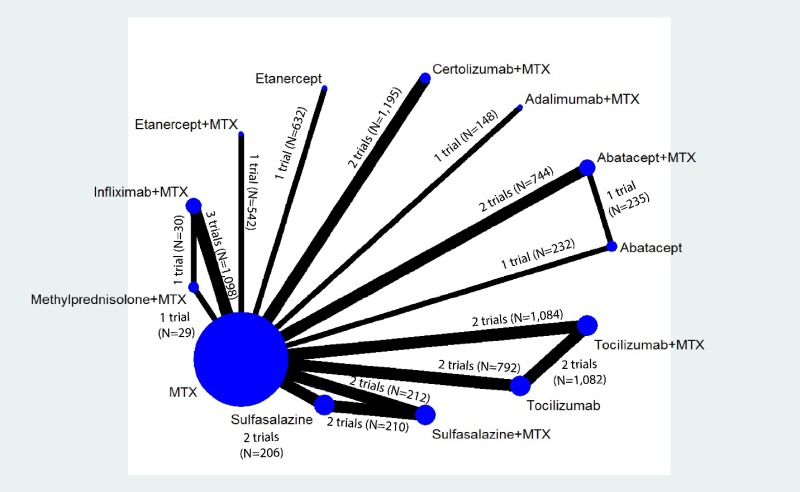
Appendix Figure H-5Network diagram for network meta-analysis: Change from baseline in radiographic joint damage score
MTX = methotrexate; N = number of patients
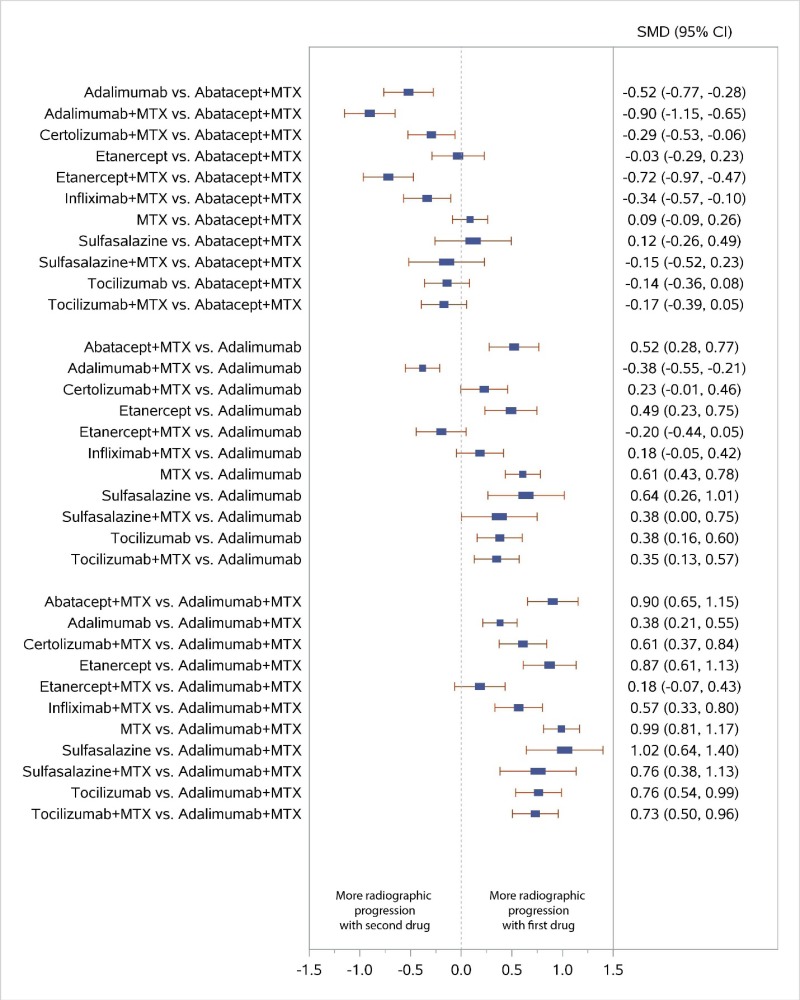
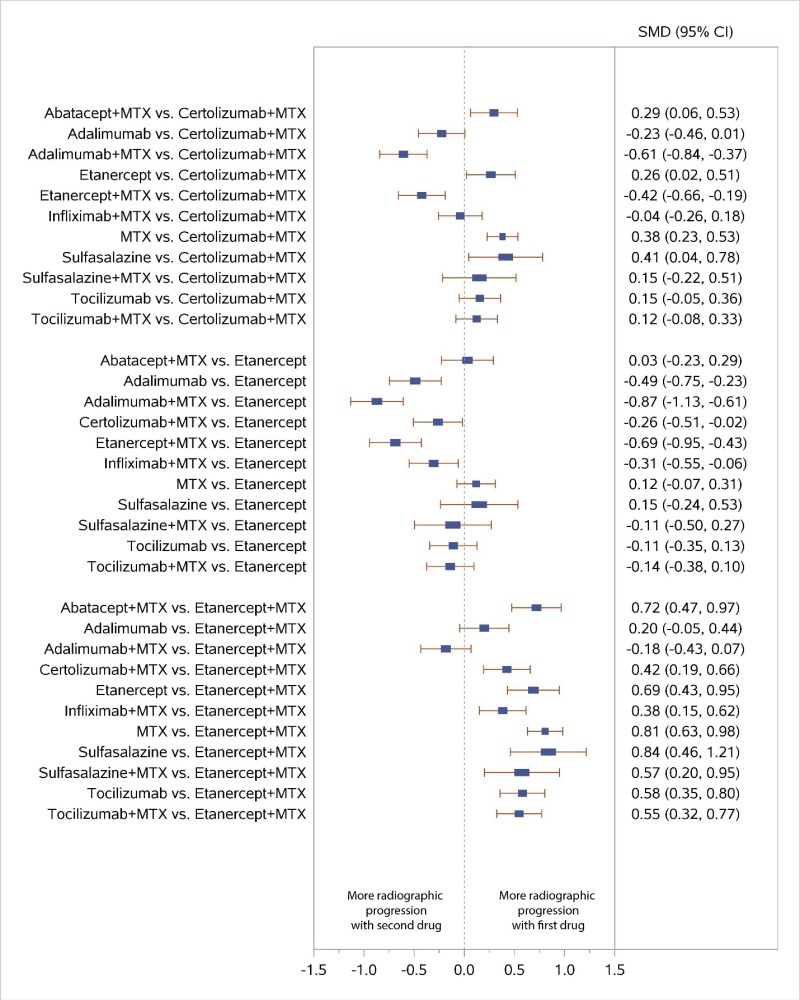
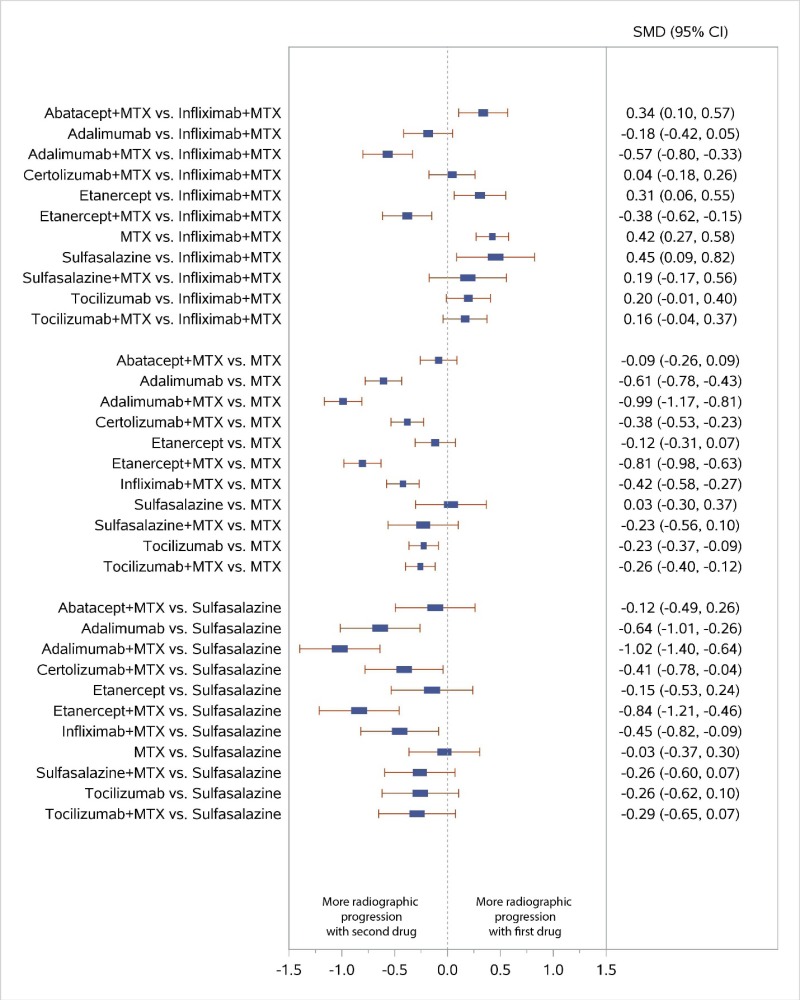
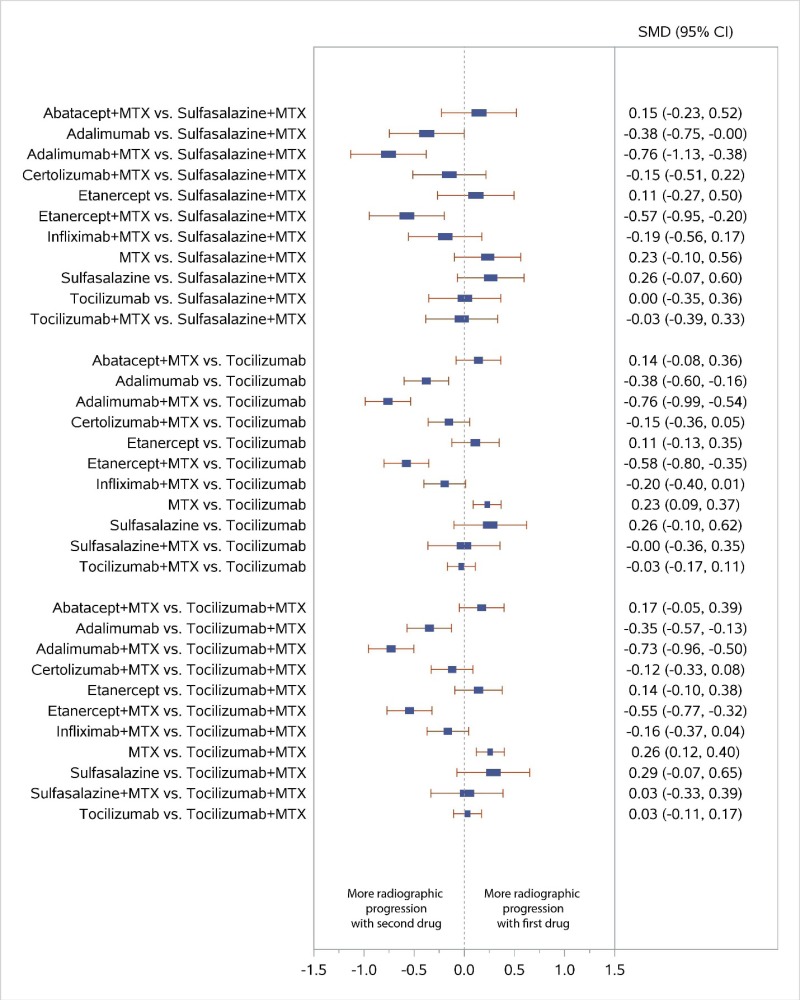
Appendix Figure H-6Forest plots for network meta-analysis: Change from baseline in radiographic joint damage score
MTX = methotrexate; SMD = standardized mean difference; vs. = versus; 95% CI = 95% confidence interval
Appendix Figure H-7Network diagram for network meta-analysis: All discontinuations and discontinuations due to adverse events
MTX = methotrexate; N = number of patients
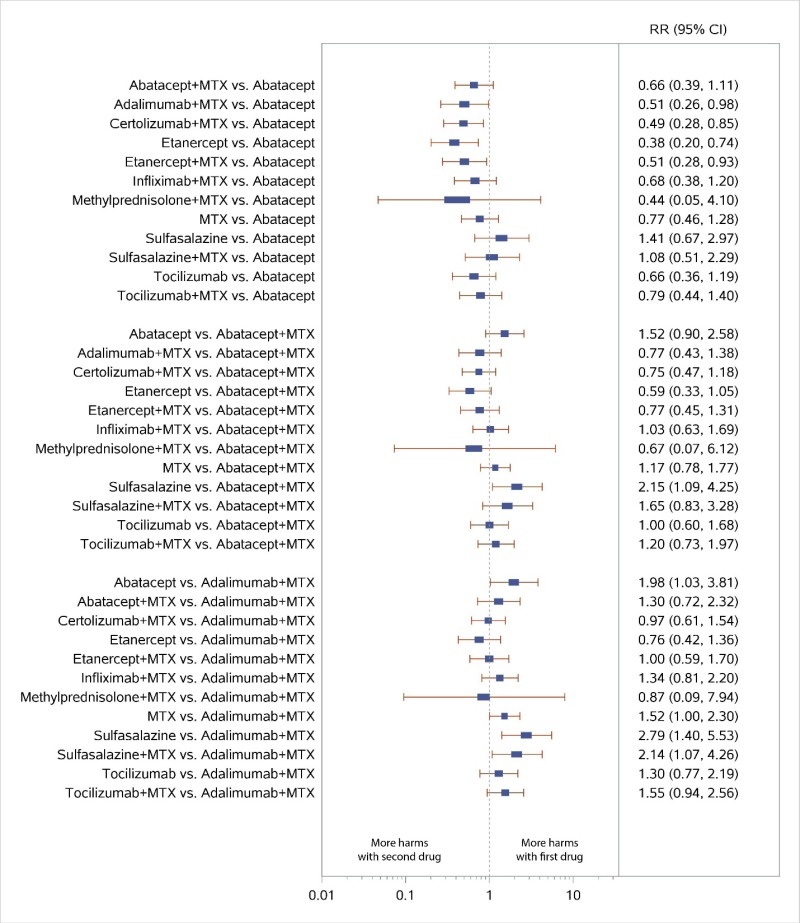
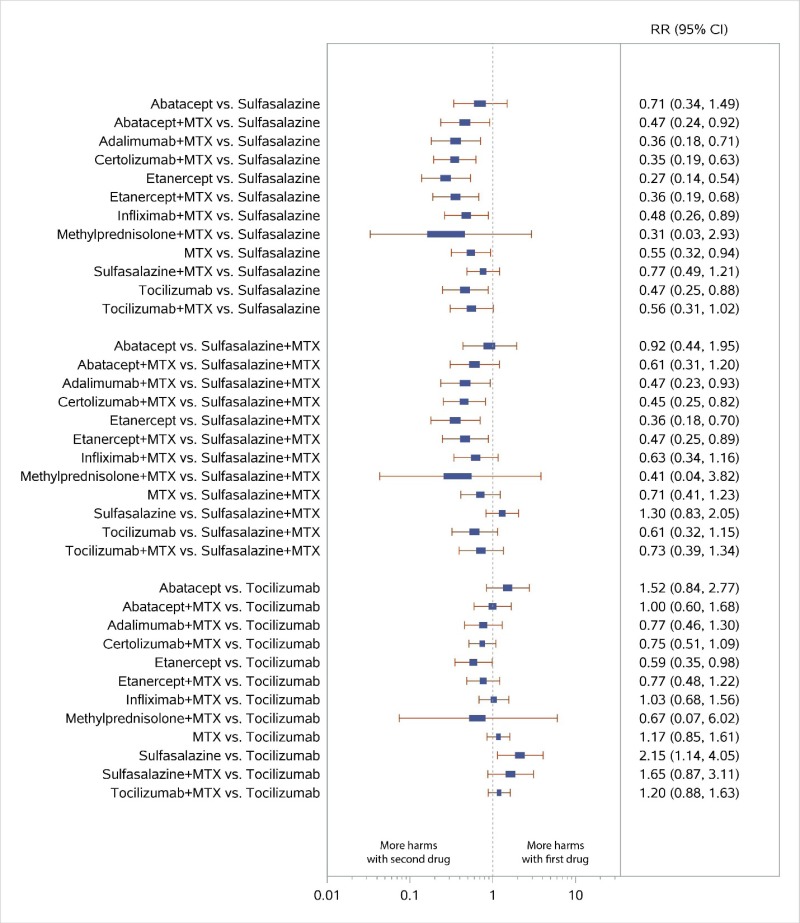
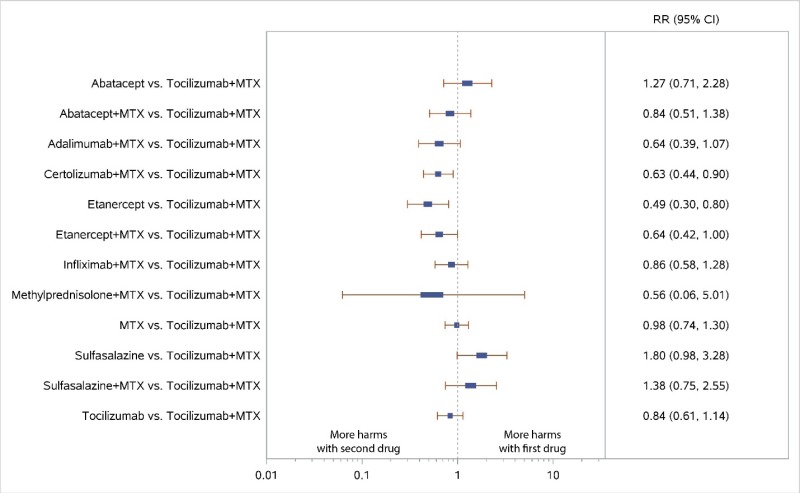
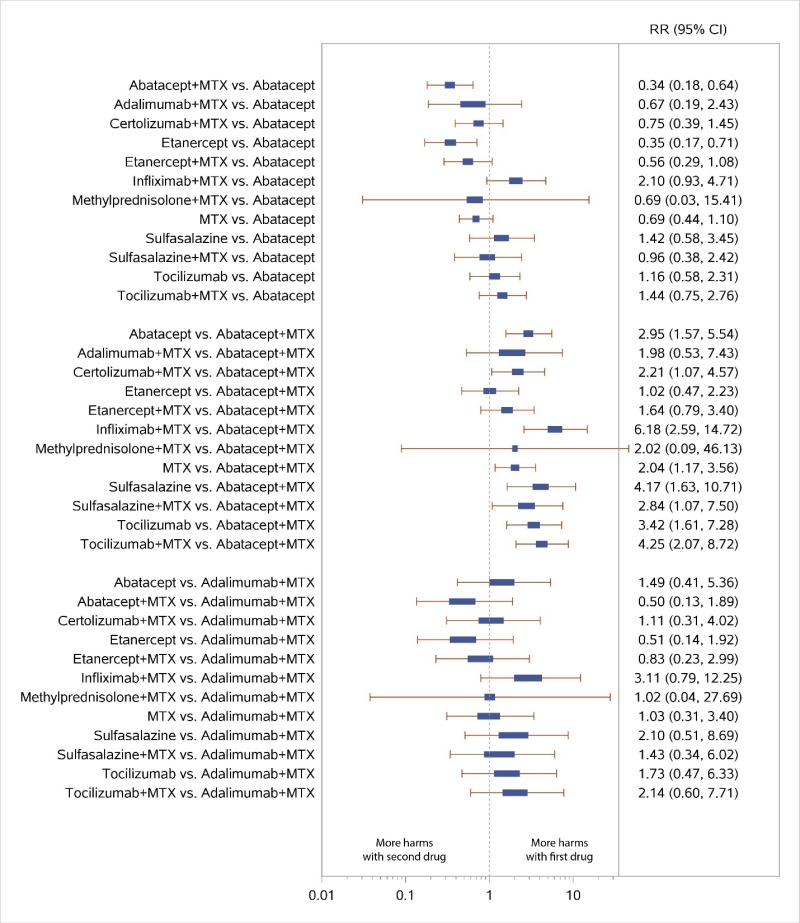
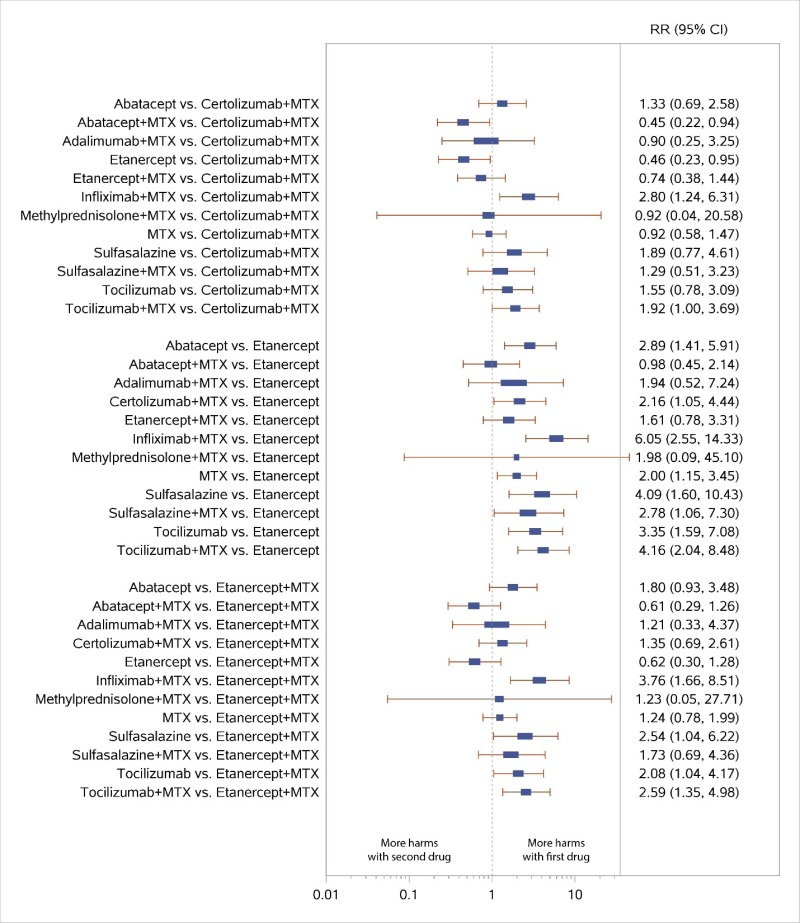
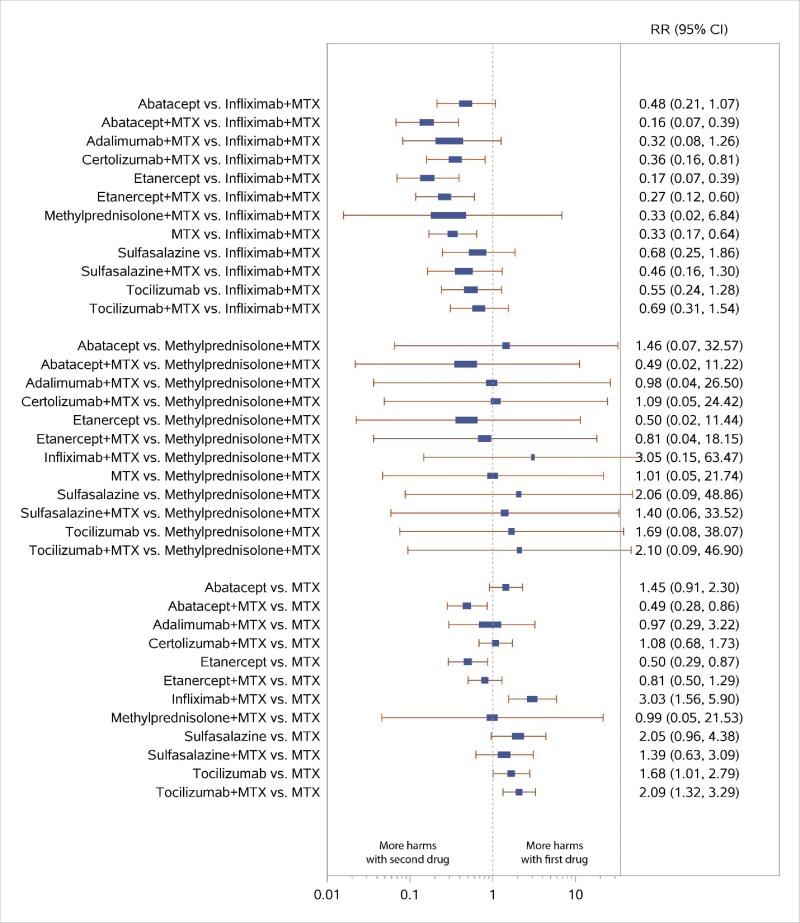
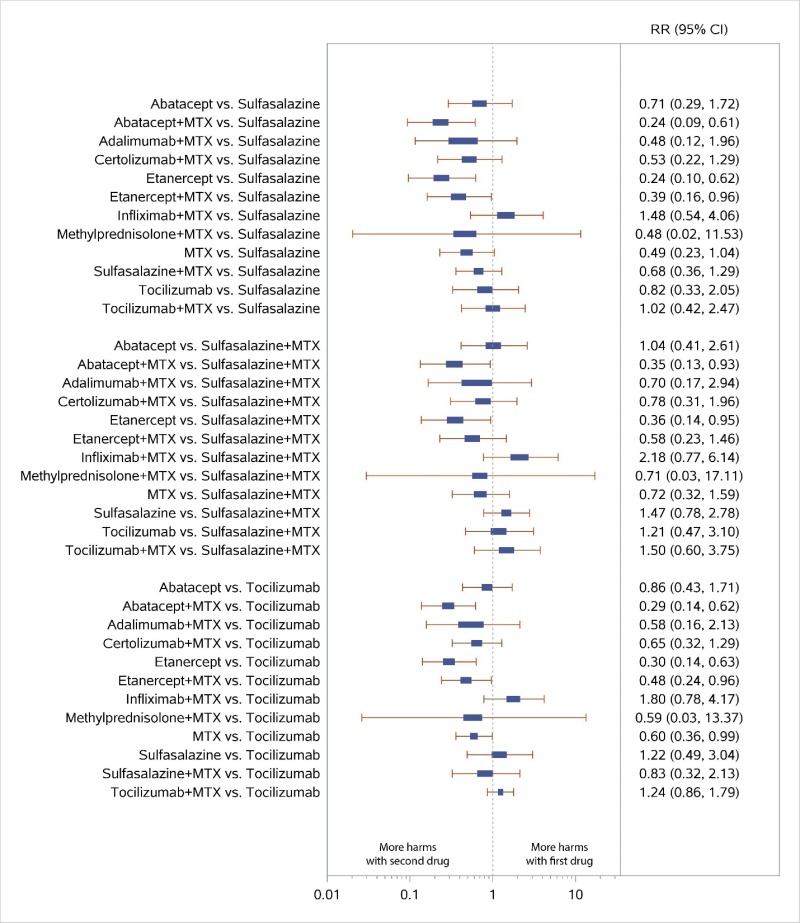
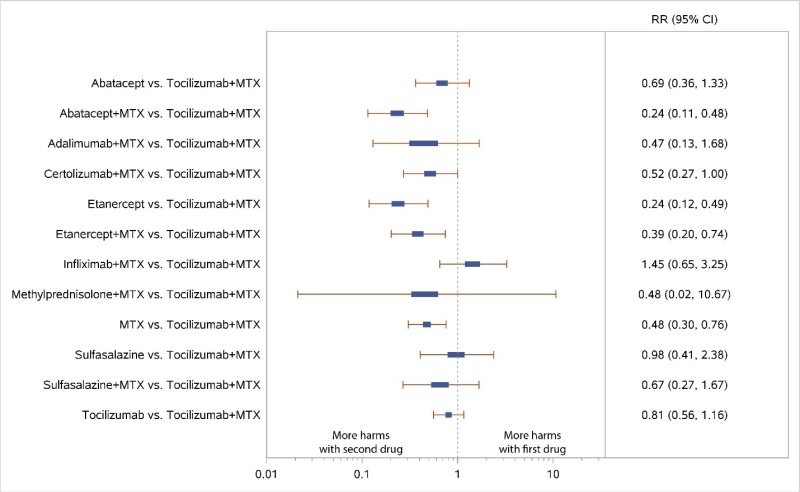
Appendix Figure H-8Forest plots for network meta-analysis: All discontinuations
MTX = methotrexate; RR = relative risk; vs. = versus; 95% CI = 95% confidence interval
- Supplementary Primary Network Meta-Analyses - Drug Therapy for Early Rheumatoid ...Supplementary Primary Network Meta-Analyses - Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update
Your browsing activity is empty.
Activity recording is turned off.
See more...