NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Donahue KE, Gartlehner G, Schulman ER, et al. Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Jul. (Comparative Effectiveness Review, No. 211.)

Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update [Internet].
Show detailsSensitivity Analyses for Network Meta-Analyses
We identified a total of 14 studies with a low or medium risk of bias for use in our main network meta-analyses (NWMA) comparing the efficacy of drug therapies for early rheumatoid arthritis. Those findings are presented in our main report.
An additional two studies provided data eligible for inclusion in these analyses but were rated as high risk of bias.13, 16 We re-ran our NWMA including these studies for our sensitivity analyses. Estimates for the treatment comparisons were very similar to estimates from our main analyses excluding those studies. We present these findings below, first for our tests of consistency and then the network diagrams and forest plots depicting effect estimates for specific drug comparisons.
Tests of Consistency: Models Including High Risk of Bias Studies
To test for consistency, we compared consistency and inconsistency models. In addition, where there were closed loops in the network diagram with both direct and indirect evidence available, we examined differences in results between direct and indirect evidence using network sidesplits.
ACR50 Response
For the ACR50 outcome (see Appendix Table I-1), there was no significant difference in the consistency and inconsistency models (χ2(3)=0.48, p=0.922). Results did not differ significantly between direct and indirect evidence for (1) Abatacept versus Abatacept plus Methotrexate (MTX) (coefficient [95% CI]= −0.09 [−0.69 to 0.52], p=0.777), (2) Adalimumab versus Adalimumab plus MTX (coefficient [95% CI]=0.17 [−0.55 to 0.89], p=0.644), or (3) Infliximab plus MTX versus Methylprednisolone plus MTX (coefficient [95% CI]= −0.37 [−1.99 to 1.25], p=0.653).
Remission According to Disease Activity Score
For the DAS outcome (see Appendix Table I-2), there was no significant difference in the consistency and inconsistency models (χ2(2)=1.66, p=0.646). Results did not differ significantly between direct and indirect evidence for (1) Abatacept versus Abatacept + MTX (coefficient (95% CI)= −0.60 (−2.09, 0.89), p=0.428), or (2) Adalimumab versus Adalimumab + MTX (coefficient (95% CI)= −0.44 (−2.56 to 1.68), p=0.685).
Appendix Table I-1Table with network sidesplits: ACR50 Response
| Drug A | Drug B | Direct Coefficient | 95% CI | p | Indirect Coefficient | 95% CI | p | Coefficient Difference | 95% CI | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Abatacept | Abatacept + MTX | 0.16 | −0.07, 0.38 | 0.178 | 0.24 | −0.33, 0.82 | 0.406 | −0.09 | −0.69, 0.52 | 0.777 |
| Adalimumab | Adalimumab + MTX | 0.42 | 0.25, 0.59 | <0.001 | 0.25 | −0.47, 0.96 | 0.503 | 0.17 | −0.55, 0.89 | 0.644 |
| Infliximab+MTX | Methylprednisolone+ MTX | 0.00 | −0.51, 0.51 | 1.000 | 0.37 | −1.17, 1.91 | 0.636 | −0.37 | −1.99, 1.25 | 0.653 |
ACR50 = American College of Rheumatology 50% response; CI = confidence interval; MTX = methotrexate
Appendix Table I-2Table with network sidesplits: Remission according to Disease Activity Score
| Drug A | Drug B | Direct Coefficient | 95% CI | p | Indirect Coefficient | 95% CI | p | Coefficient Difference | 95% CI | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Abatacept | Abatacept + MTX | 0.35 | −0.18, 0.88 | 0.192 | 0.95 | −0.45, 2.36 | 0.184 | −0.60 | −2.09, 0.89 | 0.428 |
| Infliximab+MTX | Methylprednisolone+ MTX | 0.10 | −0.56, 0.75 | 0.777 | 0.53 | −1.47, 2.54 | 0.600 | −0.44 | −2.56, 1.68 | 0.685 |
CI = confidence interval; MTX = methotrexate
Network Diagrams and Forest Plots
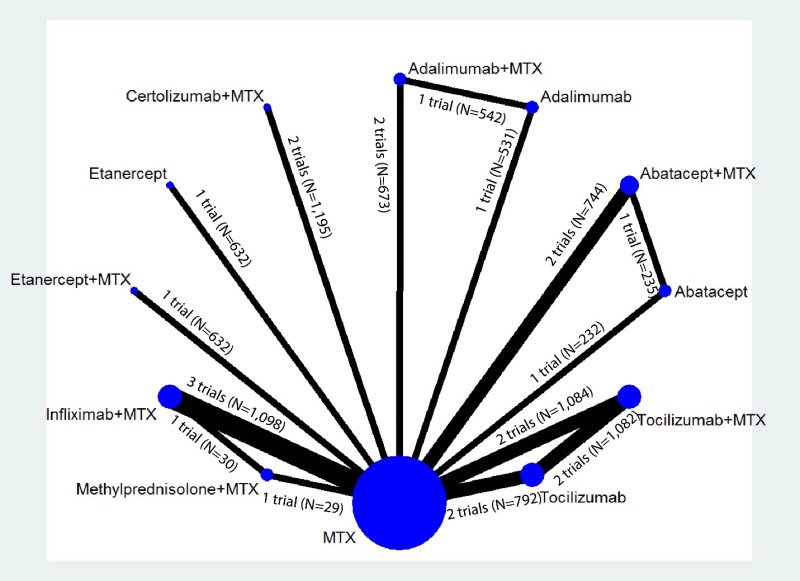
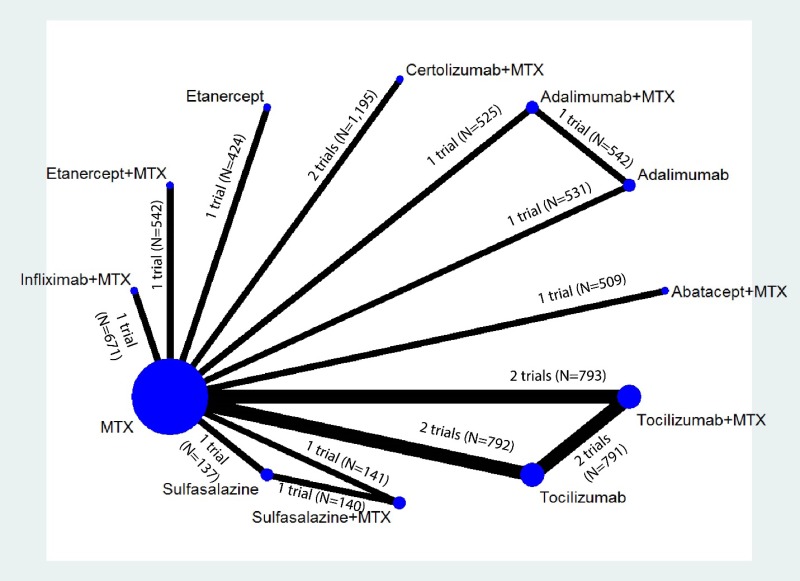
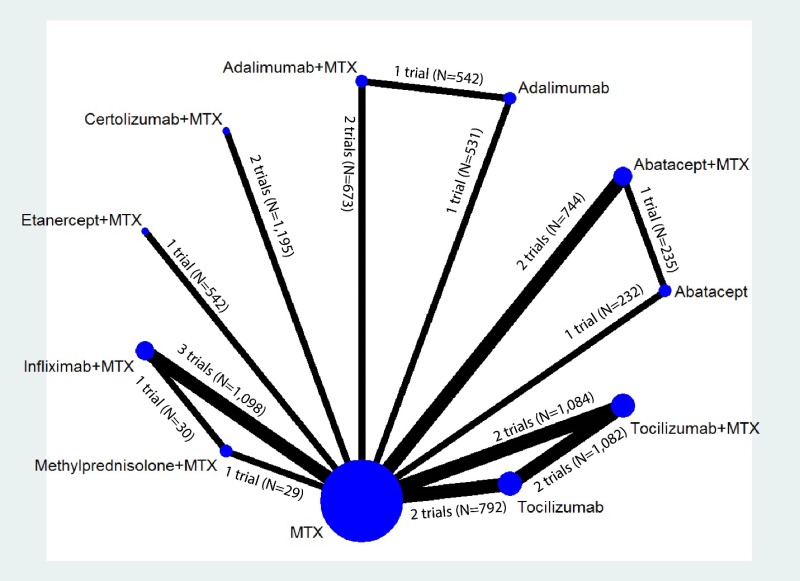
Appendix Figure I-1Network diagram for network meta-analysis (sensitivity analysis): ACR50 response
MTX = methotrexate; N = number of patients
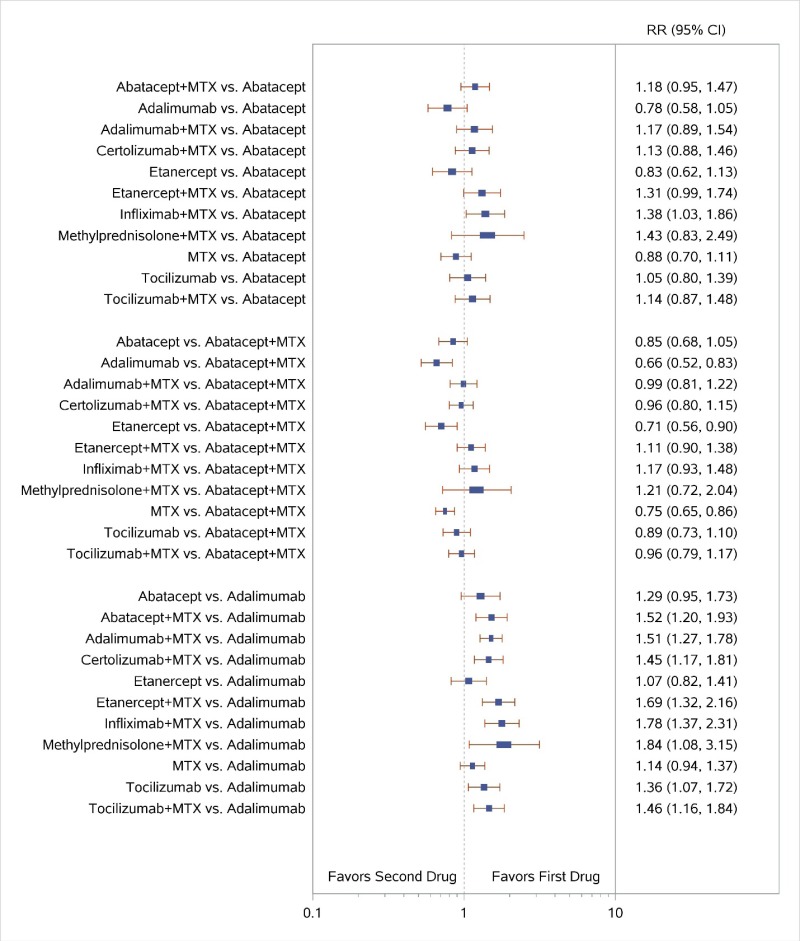
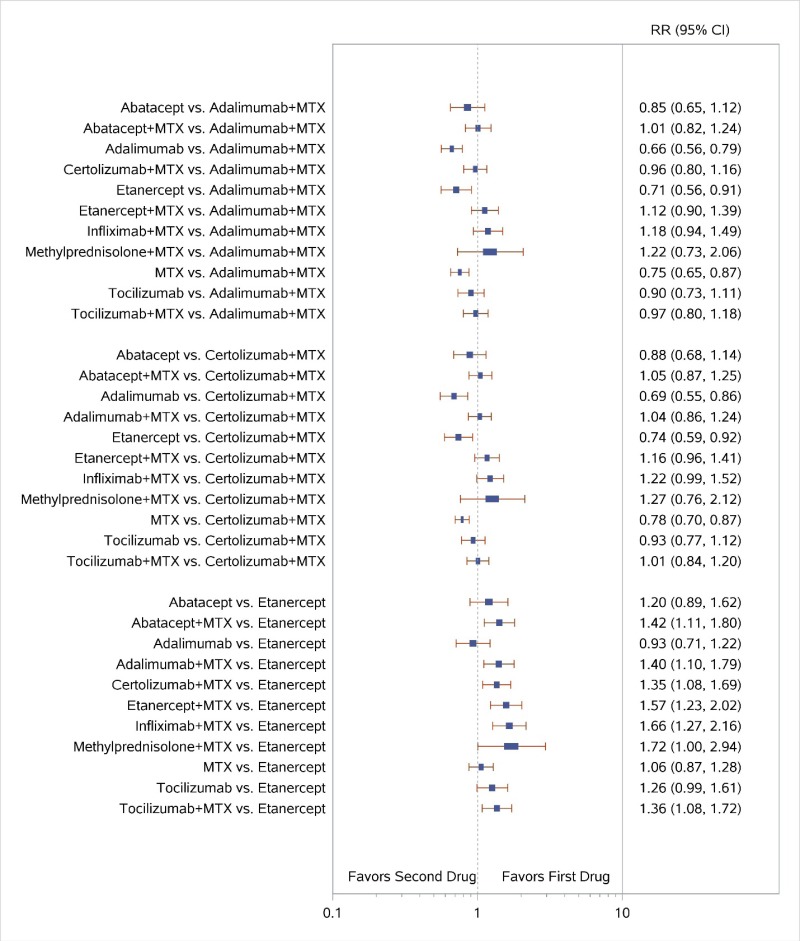
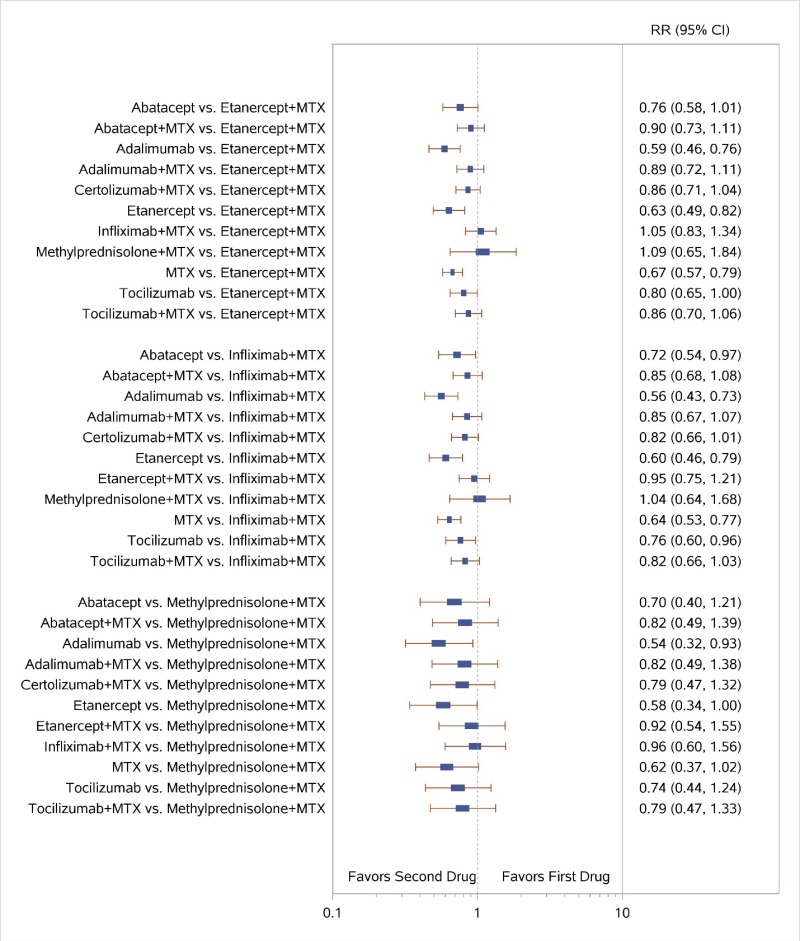
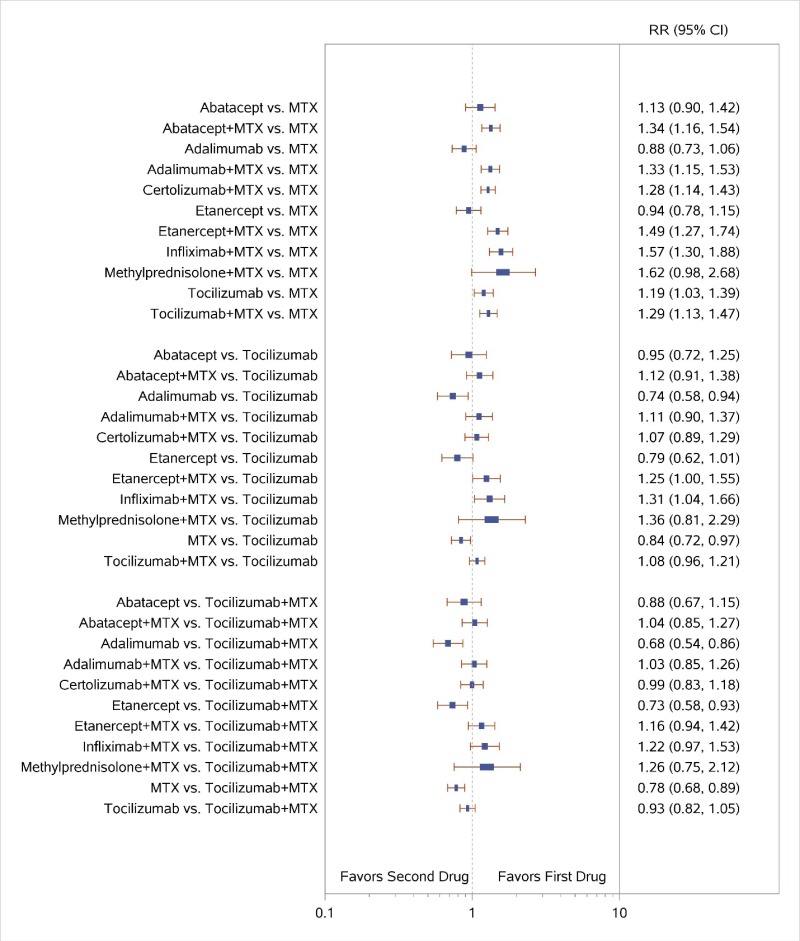
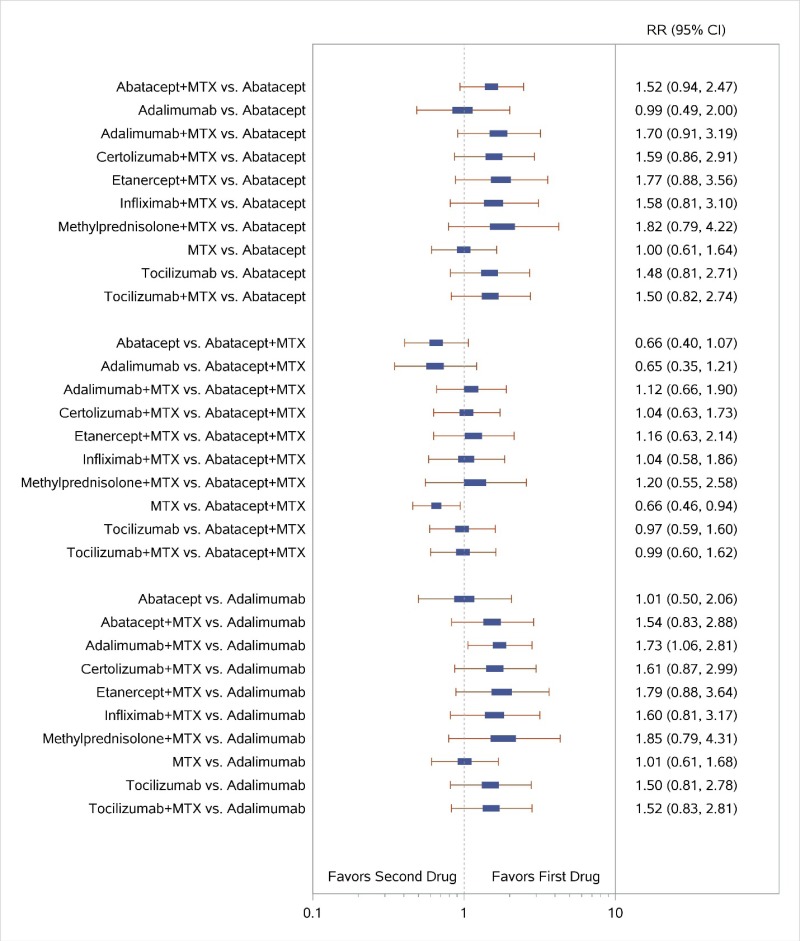
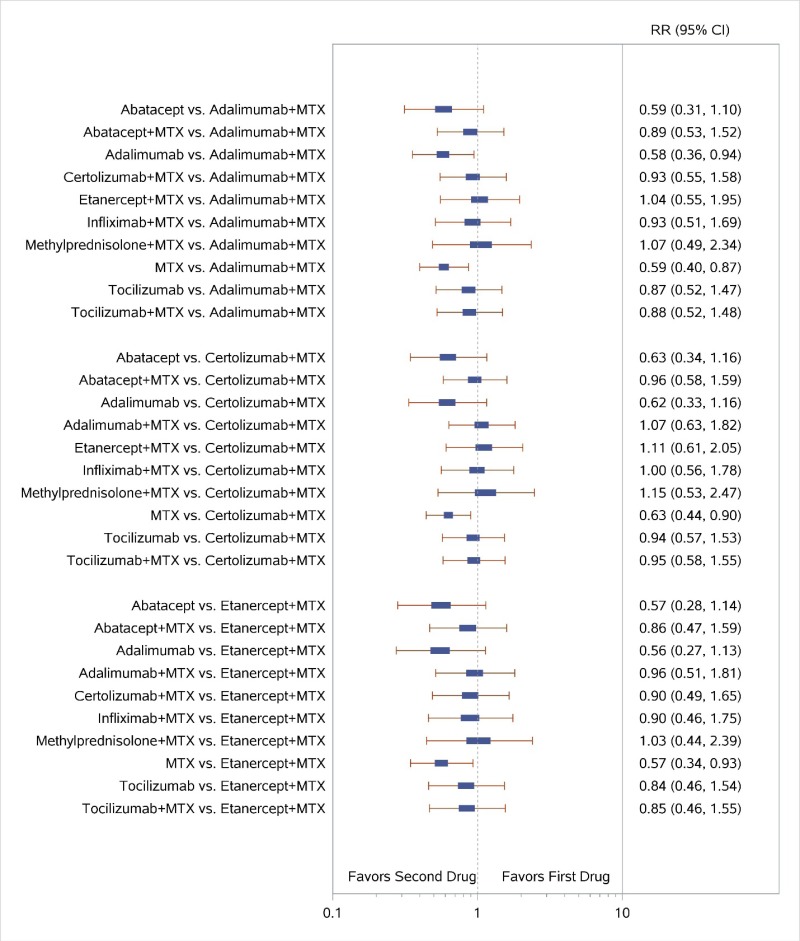
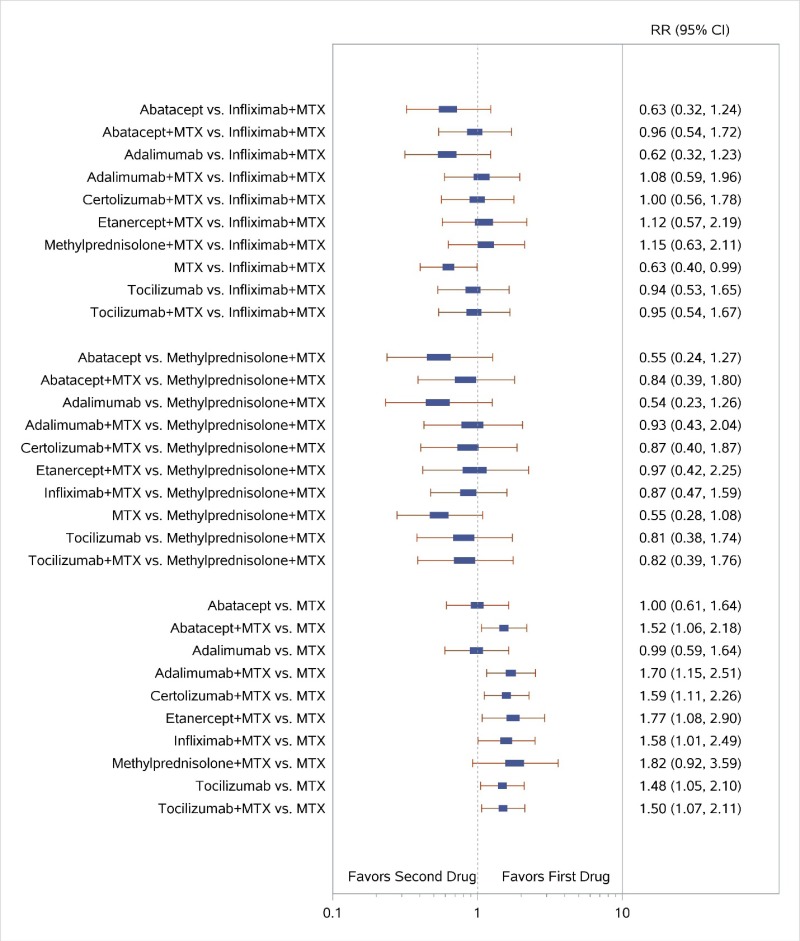
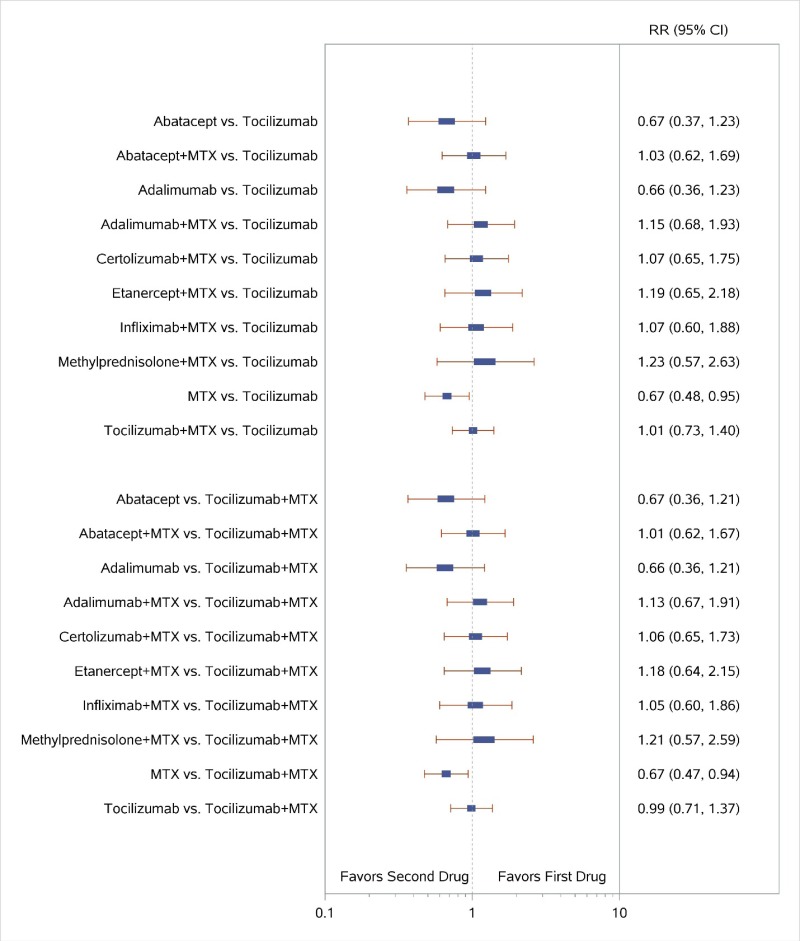
Appendix Figure I-2Forest plots for network meta-analysis (sensitivity analysis): ACR50 response
ACR50 = American College of Rheumatology 50% improvement; MTX = methotrexate; RR = relative risk; vs. = versus; 95% CI = 95% confidence interval
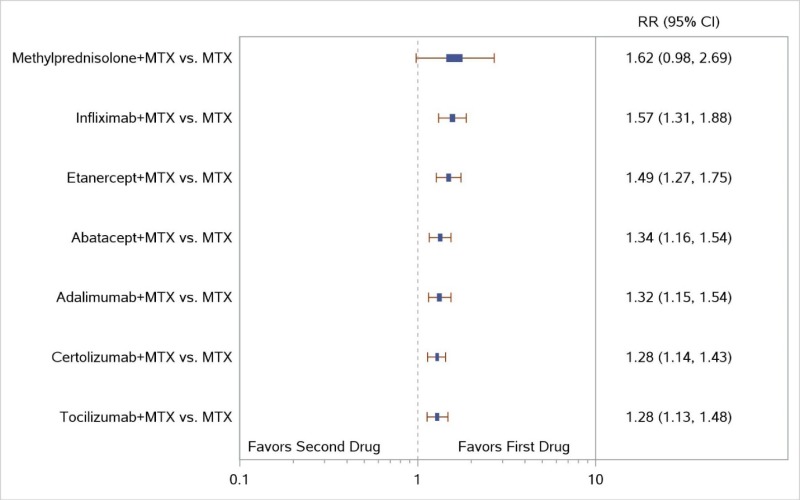
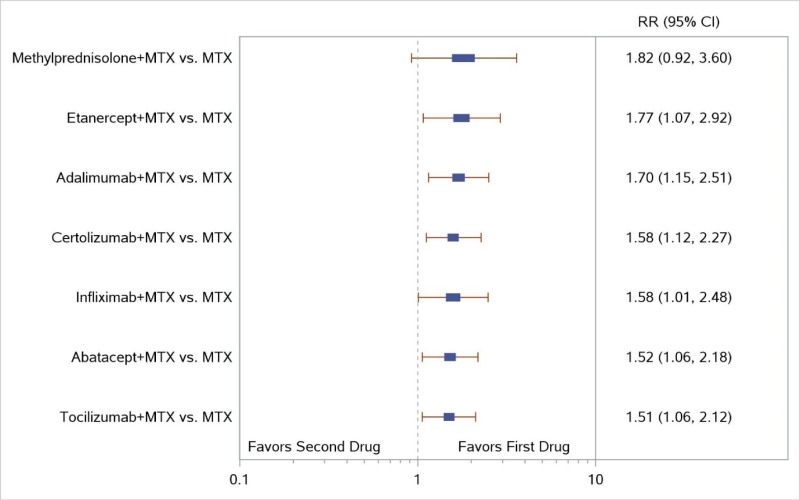
Appendix Figure I-3Forest plots for network meta-analysis (sensitivity analysis) of ACR50 response: Comparison of combined therapies to MTX only
ACR50 = American College of Rheumatology 50% improvement; MTX = methotrexate; RR = relative risk; vs. = versus; 95% CI = 95% confidence interval
Appendix Figure I-4Network diagram for network meta-analysis (sensitivity analysis): Change from baseline in radiographic joint damage score
MTX = methotrexate; N = number of patients
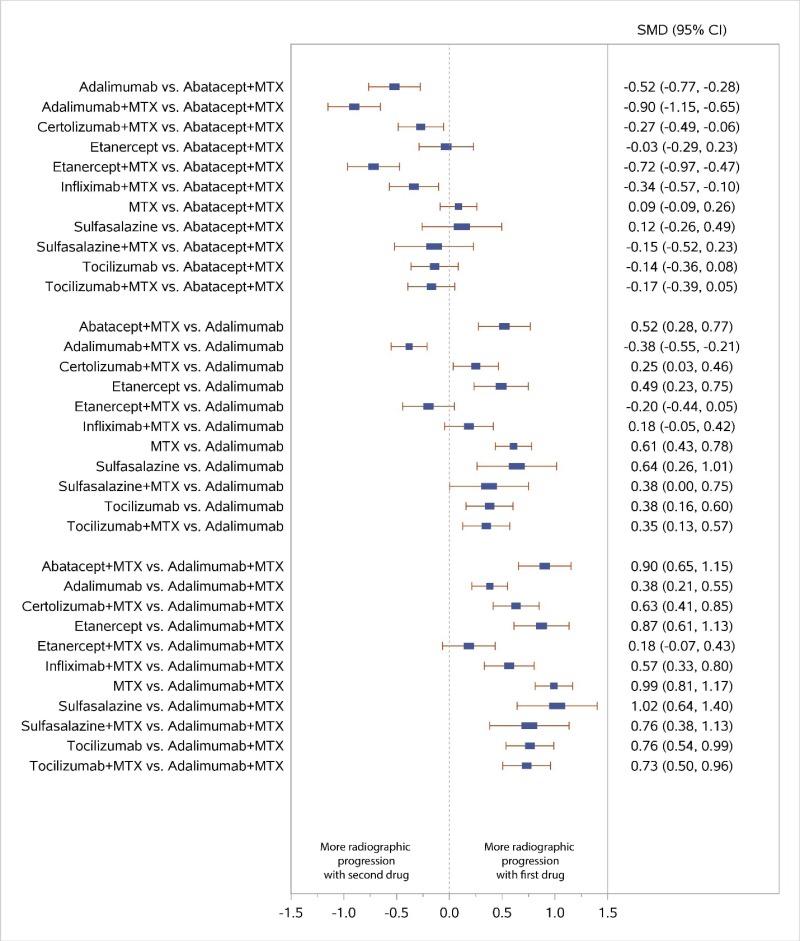
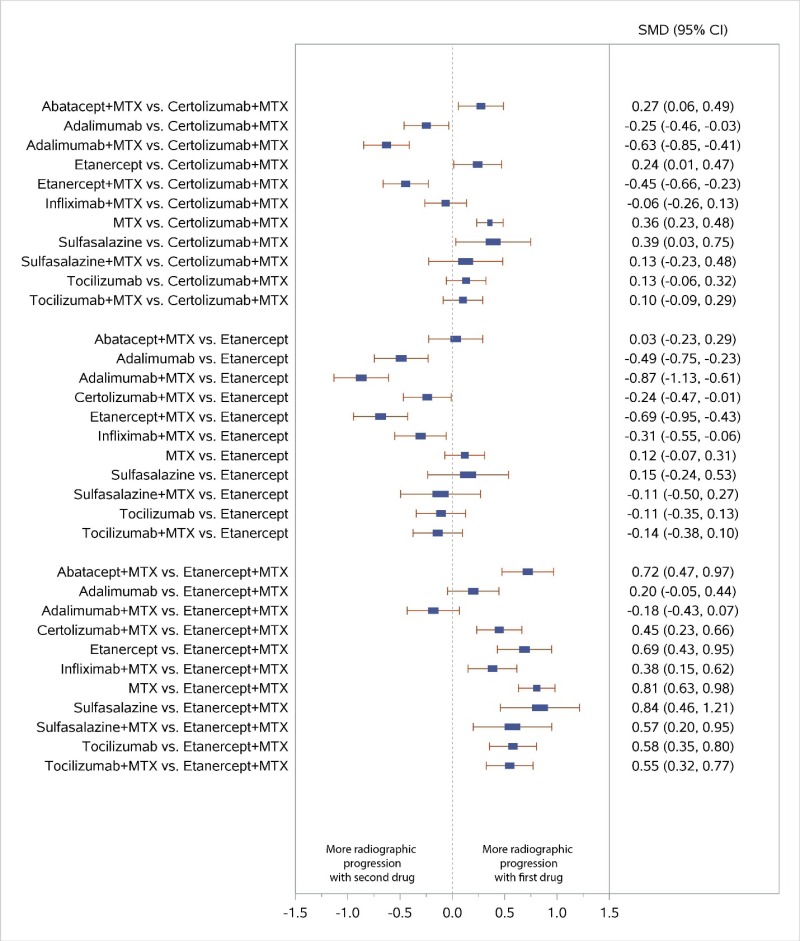
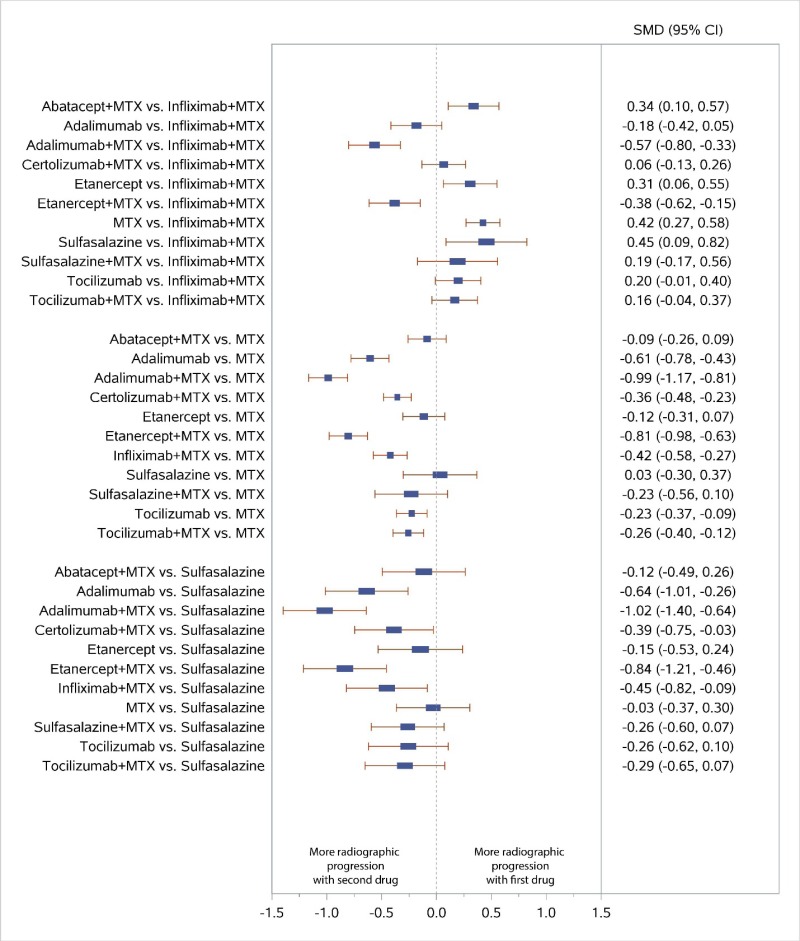
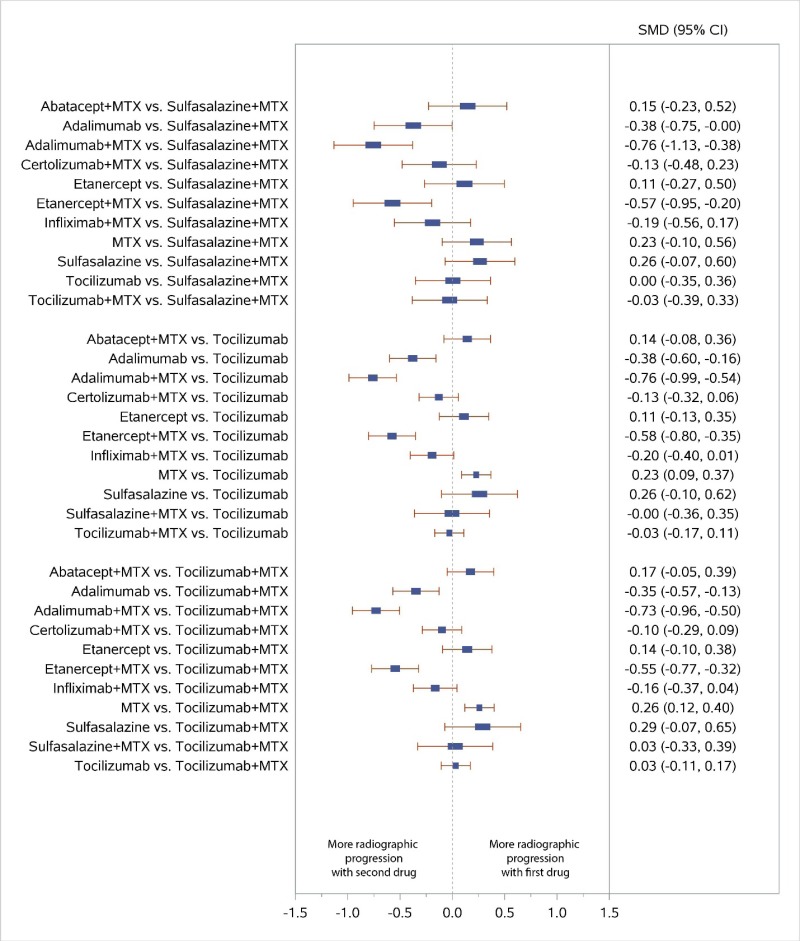
Appendix Figure I-5Forest plots for network meta-analysis (sensitivity analysis): Change from baseline in radiographic joint damage score
MTX = methotrexate; SMD = standardized mean difference; vs. = versus; 95% CI = 95% confidence interval
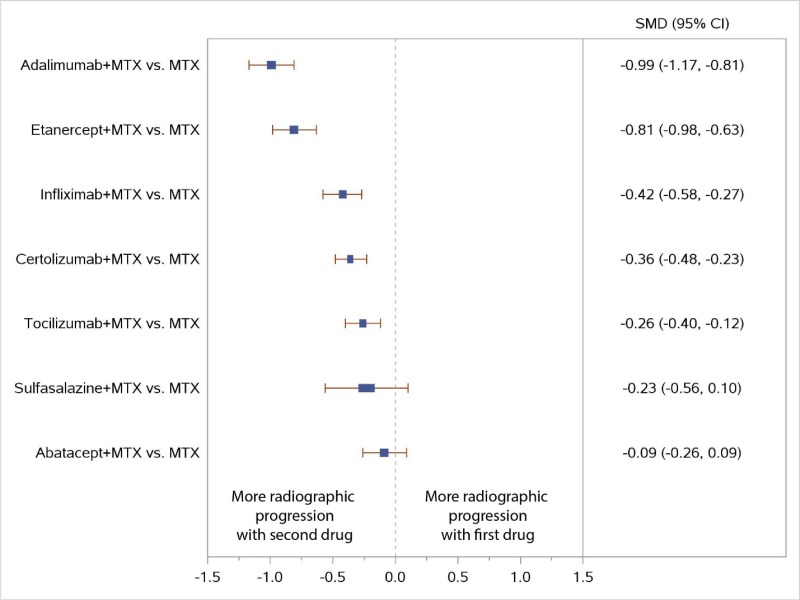
Appendix Figure I-6Forest plots for network meta-analysis (sensitivity analysis) of change from baseline in radiographic joint damage score: Comparison of combined therapies to MTX only
MTX = methotrexate; SMD = standardized mean difference; vs. = versus; 95% CI = 95% confidence interval
Appendix Figure I-7Network diagram for network meta-analysis (sensitivity analysis): Remission according to Disease Activity Score
MTX = methotrexate; N = number of patients
Appendix Figure I-8Forest plots for network meta-analysis (sensitivity analysis): Remission according to Disease Activity Score
MTX = methotrexate; RR = relative risk; vs. = versus; 95% CI = 95% confidence interval
- Sensitivity Analyses for Network Meta-Analyses - Drug Therapy for Early Rheumato...Sensitivity Analyses for Network Meta-Analyses - Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update
Your browsing activity is empty.
Activity recording is turned off.
See more...