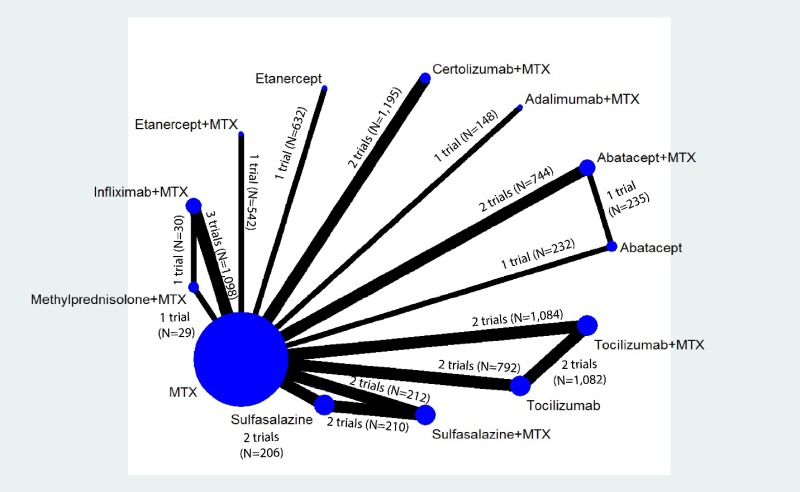
From: Appendix H, Supplementary Primary Network Meta-Analyses

Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update [Internet].
Comparative Effectiveness Review, No. 211.
Donahue KE, Gartlehner G, Schulman ER, et al.
Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Jul.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.