NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Pentamidine is a potent, broad spectrum antiinfective agent with activity against several parasitic worms, protozoa and fungi that has been used mainly in the treatment and the prophylaxis of Pneumocystis jiroveci (formerly carinii) infection in immunocompromised persons. Pentamidine is relatively toxic and therapy requires careful monitoring. Pentamidine has been associated with transient serum aminotransferase elevations during therapy and with rare instances of clinically apparent liver injury.
Background
Pentamidine (pen tam' i deen) is an aromatic diamine that is active against a broad spectrum of infectious agents. Its mechanism of action is unknown, but it appears to be taken up and concentrated within microorganisms where it inhibits DNA, RNA and protein synthesis. Pentamidine has a broad spectrum of activity against several cestodes, trematodes and protozoan parasites such as Giardia, Cryptosporidium and Entamoeba. Pentamidine is also active against Pneumocystis jiroveci (formerly known as P. carinii), which is now considered a fungal agent. Pentamidine was approved for use in the United States in 1984 at which time a major indication was the prophylaxis and therapy of Pneumocystis jiroveci infection. In intervening years, pentamidine has been replaced by better tolerated and less toxic agents in treating Pneumocystis such as trimethoprim/sulfamethoxazole or dapsone with atovaquone. Pentamidine is also an alternative, second line therapy for leishmaniasis and trypanosomiasis. Pentamidine is not absorbed well by the oral route and is available in aerosol forms (300 mg) under the brand name of Nebupent and as a solution for injection under the name Pentam 300. For treatment of pneumocystis pneumonia, the recommended regimen is 3-4 mg/kg intravenously or intramuscularly once daily for 14 to 21 days. For pneumocystis prevention, the recommended regimen is 300 mg in nebulized form every 4 weeks. Pentamidine therapy is highly toxic and the side effects of intravenous therapy are often severe and can be fatal. These side effects include nausea, abdominal discomfort, dizziness, hypotension, tachycardia, headache, rash, fever, hypoglycemia, hyponatremia, renal insufficiency and allergic reactions, including Stevens Johnson syndrome.
Hepatotoxicity
Pentamidine has been associated with serum aminotransferase elevations in 9% to 15% of patients receiving 2 to 3 weeks of therapy for pneumocystis pneumonia. Clinically apparent liver injury has also been reported with its use, but always in association with multiple other severe complications, such as respiratory or renal failure and pancreatitis. The onset of injury is within days of starting therapy and is characterized by acute hepatic necrosis, marked elevations in serum aminotransferase levels, rapid development of prolongation of prothrombin time and minimal or no jaundice. Recovery is typically rapid and usually complete.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
Pentamidine interferes with polyamine synthesis and RNA polymerase activity, which may account for its multiorgan toxicity.
Outcome and Management
Pentamidine is not very well tolerated, but its major toxicities are not hepatic, and the predominance of these other dose limiting toxicities may be the reason that liver injury is not more common. Pentamidine has not been associated with fatal acute liver failure or chronic liver injury.
Drug Class: Antifungal Agents
Pentamidine is not a typical antifungal agent and has a broad spectrum of activity that does not fit neatly into any antimicrobial class. Other agents used to treat or prevent Pneumocystis jiroveci pneumonia:
Sulfamethoxazole/Trimethoprim, Dapsone, Atovaquone, Primaquine, Clindamycin
Drug Class: Anthelmintic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pentamidine – NebuPent® [Aerosol]
DRUG CLASS
Antifungal/Anthelmintic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
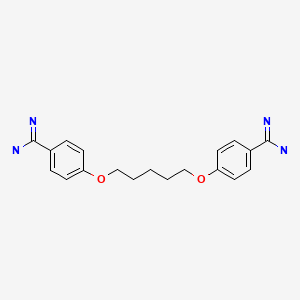
| Pentamidine | 100-33-4 | C19-H24-N4-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 July 2020
- Zimmerman HJ. Antifungal agents. Hepatic injury from antimicrobial agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 609-11.(Expert review of hepatotoxicity of antifungal agents written in 1999; severe cases of liver injury have been reported in patients with AIDS receiving pentamidine).
- Wetzel DM, Phillips MA. Chemotherapy of protozoal infections: amebiasis, giardiasis, trichomoniasis, trypanosomiasis, leishmaniasis, and other protozoal infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 985-99.(Textbook of pharmacology and therapeutics).
- National Library of Medicine. , NIH. http://aidsinfo
.nih.gov/guidelines/ [PubMed: 28792816] (Web site with recent guidelines on management and prevention of opportunistic infections including P. jiroveci in persons with HIV infection). - Waalkes TP, Denham C, Devita VT. Pentamidine: clinical pharmacologic correlations in man and mice. Clin Pharmacol Ther. 1970;11:505–12. [PubMed: 5310706](Analysis of pharmacokinetics and toxicity of pentamidine in 7 patients with cancer being treated for P. jiroveci pneumonia; minor changes in liver enzymes were noted in some patients).
- Western KA, Perera DR, Schultz MG. Pentamidine isethionate in the treatment of Pneumocystis carinii pneumonia. Ann Intern Med. 1970;73:695–702. [PubMed: 5312203](Among 164 patients with P. jiroveci pneumonia treated with pentamidine, 42% had side effects including 12 [7%] with abnormal liver tests; AST 48-170 U/L, but 1 patient had jaundice and AST 2000 2 weeks after stopping, ultimately resolving at least in part ).
- Picon M, Causse X, Gelas P, Retornaz G, Trépo C, Bouletreau P. Gastroenterol Clin Biol. 1991;15:463–4. [Pentamidine-related acute hepatitis during pneumocystosis treatment in acquired immunodeficiency syndrome] French. [PubMed: 2070975](27 year old with HIV infection and severe P. jiroveci pneumonia developed renal insufficiency, pancreatitis [amylase 623 U/L] and acute hepatic necrosis after 6 days of intravenous pentamidine, with ALT 2000 U/L and decrease in prothrombin time with rapid recovery upon stopping).
- Balslev U, Nielsen TL. Adverse effects associated with intravenous pentamidine isethionate as treatment of Pneumocystis carinii pneumonia in AIDS patients. Dan Med Bull. 1992;39:366–8. [PubMed: 1526188](Retrospective analysis of side effects of pentamidine therapy in 21 patients with AIDS and P. jiroveci infection; serum enzyme elevations occurred in 3 patients but were minor; major side effects [n=5] included hypoglycemia, pancreatitis and cardiac arrest).
- O'Brien JG, Dong BJ, Coleman RL, Gee L, Balano KB. A 5-year retrospective review of adverse drug reactions and their risk factors in human immunodeficiency virus-infected patients who were receiving intravenous pentamidine therapy for Pneumocystis carinii pneumonia. Clin Infect Dis. 1997;24:854–9. [PubMed: 9142782](Retrospective analysis of adverse drug reactions to intravenous pentamidine therapy of Pneumocystis jiroveci pneumonia, identified 174 events in 72% of 106 patients treated with pentamidine; most common were nephrotoxicity [48%], hypoglycemia [24%], hyperkalemia [19%] and serum enzyme elevations [15%]).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, only one of which was attributed to an anthelmintic, mebendazole; none were attributed to pentamidine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 409 [46%] were attributed to antimicrobial agents, but none to anthelmintics or to pentamidine ).
- Pohlig G, Bernhard SC, Blum J, Burri C, Mpanya A, Lubaki JP, Mpoto AM, et al. Efficacy and safety of pafuramidine versus pentamidine maleate for treatment of first stage sleeping sickness in a randomized, comparator-controlled, international phase 3 clinical trial. PLoS Negl Trop Dis. 2016;10:e0004363. [PMC free article: PMC4755561] [PubMed: 26882015](Among 273 patients with human African trypanosomiasis treated with pafuramidine or pentamidine, cure rates were higher with pentamidine [95% vs 89%] as were ALT elevations [52% vs 1.5%]; no mention of hepatotoxicity).
- Solodokin LJ, Klejmont LM, Scipione MR, Dubrovskaya Y, Lighter-Fisher J, Papadopoulos J. Safety and effectiveness of intravenous pentamidine for prophylaxis of pneumocystis jirovecii pneumonia in pediatric hematology/oncology patients. J Pediatr Hematol Oncol. 2016;38:e180–5. [PubMed: 27164533](Among 121 children with hematologic malignancies treated with intravenous pentamidine as prophylaxis, none developed pneumocystis jirovecii infection and 19 [16%] developed adverse events that led to discontinuation in 5 [4%], but none were hepatic related, and there were no “clinically significant changes in blood chemistry levels”).
- Huang YS, Yang JJ, Lee NY, Chen GJ, Ko WC, Sun HY, Hung CC. Treatment of Pneumocystis jirovecii pneumonia in HIV-infected patients: a review. Expert Rev Anti Infect Ther. 2017;15:873–92. [PubMed: 28782390](Review of pneumocystis pneumonia in HIV-infected patients and its prevention and management, trimethoprim-sulfamethoxazole being the treatment of choice, pentamidine being a second line option, but which requires slow intravenous administration and which has many adverse events most commonly renal injury, hyperkalemia, hypoglycemia, neutropenia and torsades de pointes; no mention of ALT elevations or hepatotoxicity).
- Sweiss K, Anderson J, Wirth S, Oh A, Quigley JG, Khan I, Saraf S, et al. A prospective study of intravenous pentamidine for PJP prophylaxis in adult patients undergoing intensive chemotherapy or hematopoietic stem cell transplant. Bone Marrow Transplant. 2018;53:300–6. [PubMed: 29269796](Among 50 adult patients with hematologic malignancies undergoing chemotherapy or hematopoietic cell transplantation treated with prophylactic intravenous pentamidine [4 mg/kg] monthly, none developed pneumocystis pneumonia and there were no severe adverse events, although therapy was temporarily interrupted in 2 subjects [one for nausea and one for facial numbness] and there was no mention of ALT elevations or hepatotoxicity).
- Gadelha EPN, Ramasawmy R, da Costa Oliveira B, Morais Rocha N, de Oliveira Guerra JA, Allan Villa Rouco da Silva G, Gabrielle Ramos de Mesquita T, et al. An open label randomized clinical trial comparing the safety and effectiveness of one, two or three weekly pentamidine isethionate doses (seven milligrams per kilogram) in the treatment of cutaneous leishmaniasis in the Amazon Region. PLoS Negl Trop Dis. 2018;12(10):e0006850. [PMC free article: PMC6231690] [PubMed: 30379814](Among 159 patients with American Cutaneous Leishmaniasis treated with 1, 2 or 3 weekly infusions of pentamidine [7 mg/kg], cure rates were 45%, 81% and 96% and side effects were common but mild and did not result in any early discontinuations; no mention of ALT elevations or hepatotoxicity).
- Wilcox MA, Hardin J, Weaver J, Voss EA. Liver test monitoring: real-world compliance for drugs with monitoring requirements at 2-week intervals or more frequently. Pharmaceut Med. 2019;33:389–94. [PubMed: 31933226](Analysis of 3 large health databases for compliance with recommendations for liver test monitoring when initiating therapy with 9 drugs found compliance was highest for oxaliplatin [75%], somewhat lower for rifampin [68%], tolcapone [67%], albendazole [66%] and azathioprine [61%], and poor for pentamidine [21%], felbamate [22%], succimer [29%] and ketoconazole [32%]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- [Pneumocystis carinii infection during prophylaxis with nebulized pentamidine in a patient with AIDS].[Ned Tijdschr Geneeskd. 1991][Pneumocystis carinii infection during prophylaxis with nebulized pentamidine in a patient with AIDS].de Jong MD, Lange JM, Smits NJ, Reiss P. Ned Tijdschr Geneeskd. 1991 Mar 9; 135(10):424-7.
- Intravenous pentamidine for Pneumocystis carinii/jiroveci pneumonia prophylaxis in pediatric transplant patients.[Pediatr Transplant. 2015]Intravenous pentamidine for Pneumocystis carinii/jiroveci pneumonia prophylaxis in pediatric transplant patients.Clark A, Hemmelgarn T, Danziger-Isakov L, Teusink A. Pediatr Transplant. 2015 May; 19(3):326-31. Epub 2015 Feb 25.
- Increased Pneumocystis carinii recovery from the upper lobes in Pneumocystis pneumonia. The effect of aerosol pentamidine prophylaxis.[Chest. 1993]Increased Pneumocystis carinii recovery from the upper lobes in Pneumocystis pneumonia. The effect of aerosol pentamidine prophylaxis.Baughman RP, Dohn MN, Shipley R, Buchsbaum JA, Frame PT. Chest. 1993 Feb; 103(2):426-32.
- Review Widespread dissemination of Pneumocystis carinii infection in a patient with acquired immune deficiency syndrome receiving long-term treatment with aerosolized pentamidine.[Am J Clin Pathol. 1991]Review Widespread dissemination of Pneumocystis carinii infection in a patient with acquired immune deficiency syndrome receiving long-term treatment with aerosolized pentamidine.Dembinski AS, Smith DM, Goldsmith JC, Woods GL. Am J Clin Pathol. 1991 Jan; 95(1):96-100.
- Review Update on pentamidine for the treatment of Pneumocystis carinii pneumonia.[Clin Pharm. 1988]Review Update on pentamidine for the treatment of Pneumocystis carinii pneumonia.Salamone FR, Cunha BA. Clin Pharm. 1988 Jul; 7(7):501-10.
- Pentamidine - LiverToxPentamidine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...