NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Pentazocine is a synthetic opioid with both agonist and antagonist activity against opiate receptors which was previously used in oral and parenteral forms as an analgesic for moderate-to-severe pain. Currently, it is only available in tablet form in combination with either acetaminophen or naloxone. Pentazocine has not been linked to serum enzyme elevations during therapy or to clinically apparent liver injury.
Background
Pentazocine (pen taz’ oh seen) is a synthetic opioid that has both partial agonist and antagonist activity and is similar to butorphanol. Pentazocine has weak antagonist or partial agonist activity to the µ type opiate receptors, with full agonist activity at the ĸ opioid receptor. These actions lead to typical analgesic effects of the opioids at low doses, but with a dysphoric effect at higher doses, which is believed to limit its abuse potential and puts a ceiling on its analgesic effect. Pentazocine was first approved for use in the United States in 1967 for treatment of mild-to-moderate pain but is now rarely used. Oral formulations of pentazocine are available only in fixed combinations (50 mg) with acetaminophen (Telacen and generics) or naloxone (Talwin Nx and generics). The addition of acetaminophen or naloxone is believed to prevent or discourage injection and abuse of pentazocine (the naloxone being an opiate antagonist that is active only with parenteral administration). Side effects of pentazocine include sedation, respiratory depression, confusion, euphoria, agitation, itching, sweating, abdominal bloating, nausea, vomiting and constipation, adverse effects which are typical of the opioids. In addition, pentazocine can cause confusion, disorientation and hallucinations. Pentazocine is a controlled substance and is classified as a Schedule IV drug, indicating that it has medical usefulness and a mild potential for physical and psychological dependency and abuse. Internationally and in some states, pentazocine is classified as a Schedule III drug, indicating a greater potential for dependency and abuse.
Hepatotoxicity
Therapy with pentazocine has not been linked to serum enzyme elevations or to idiosyncratic acute, clinically apparent liver injury.
Likelihood score: E (unlikely cause of drug-induced liver injury).
References on the safety and potential hepatotoxicity of pentazocine are given in the Overview section of the Opioids.
Drug Class: Opioids
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pentazocine – Generic, Talwin®
DRUG CLASS
Opioids
Product labeling at DailyMed, National Library of Medicine, NIH
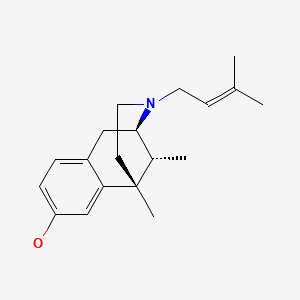
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Pentazocine | 359-83-1 | C19-H27-N-O |
|
- PubChem SubstanceRelated PubChem Substances
- Review Opioids.[LiverTox: Clinical and Researc...]Review Opioids.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Opioid agonist-antagonist drugs in acute and chronic pain states.[Drugs. 1991]Review Opioid agonist-antagonist drugs in acute and chronic pain states.Hoskin PJ, Hanks GW. Drugs. 1991 Mar; 41(3):326-44.
- Precipitated withdrawal by pentazocine in methadone-maintained volunteers.[J Pharmacol Exp Ther. 1993]Precipitated withdrawal by pentazocine in methadone-maintained volunteers.Strain EC, Preston KL, Liebson IA, Bigelow GE. J Pharmacol Exp Ther. 1993 Nov; 267(2):624-34.
- Acute effects of pentazocine, naloxone and morphine in opioid-dependent volunteers.[J Pharmacol Exp Ther. 1994]Acute effects of pentazocine, naloxone and morphine in opioid-dependent volunteers.Lamas X, Farre M, Cami J. J Pharmacol Exp Ther. 1994 Mar; 268(3):1485-92.
- Pharmacological study of pentazocine-naloxone combination: interest as a potentially non abusable oral form of pentazocine.[Arch Int Pharmacodyn Ther. 1984]Pharmacological study of pentazocine-naloxone combination: interest as a potentially non abusable oral form of pentazocine.Legros J, Khalili-Varasteh H, Margetts G. Arch Int Pharmacodyn Ther. 1984 Sep; 271(1):11-21.
- Pentazocine - LiverToxPentazocine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...