NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Clindamycin is a broad spectrum antibiotic used orally, topically and parenterally for bacterial infections due to sensitive organisms. Clindamycin has been linked to rare instances of acute liver injury.
Background
Clindamycin (klin" da mye' sin) is a lincomycin derivative with activity against many aerobic gram-positive cocci as well as many anaerobic gram-negative and gram-positive organisms. It has special activity against Bacteroides fragilis and some activity against Toxoplasma gondii and Pneumocystis jiroveci. Clindamycin acts by its binding to the 50S ribosomal subunit of bacteria, thus inhibiting protein synthesis. Clindamycin was approved for use in the United States in 1970 and is still in wide use with several million prescriptions being filled yearly. Current indications include moderate-to-severe bacterial infections caused by sensitive organisms. It is also used topically for acne and bacterial vaginosis. Clindamycin is available generically in oral and parenteral forms and as gels, foam, lotion and creams for topical use. Oral formulations include capsules of 75, 150 and 300 mg that are available in generic forms and under the commercial name of Cleocin. Clindamycin is also available in suspension for pediatric use. The typical adult dose is 600 to 2700 mg im or iv daily (in two divided doses) or 150 to 450 mg orally every 6 hours for 5 to 14 days, depending upon the type and severity of infection. Common side effects include nausea, diarrhea, headache and skin rash.
Hepatotoxicity
Clindamycin has been linked to two forms of hepatotoxicity: transient serum aminotransferase elevations usually occurring after several days of high intravenous doses; and, an acute, idiosyncratic liver injury that arises within 1 to 3 weeks of starting therapy and is typically mild and self-limited.
High doses of intravenous clindamycin can be accompanied by elevations in serum ALT levels in the range of 2 to 10 times the upper limit of normal starting after 5 to 15 days of therapy in a manner similar to what occurs with intravenous oxacillin therapy (Case 1). Symptoms, jaundice, and alkaline phosphatase elevations are mild if they occur at all (Case 2), and aminotransferase levels rapidly fall into the normal range (in 1 to 2 weeks) upon stopping clindamycin or switching to lower doses or to oral formulations with which it rarely occurs.
Clindamycin therapy has also been linked to a clinically apparent, idiosyncratic liver injury that arises between 1 to 3 weeks after starting either oral or parenteral therapy (Case 3). The pattern of serum enzyme elevations is typically hepatocellular or mixed, but can be cholestatic. Allergic manifestations such as rash, fever and eosinophilia are typical, but often are not prominent and are not present in all cases. Autoantibodies are generally not present. The acute liver injury may accompany other signs of hypersensitivity such as Stevens Johnson syndrome or other severe skin reactions. The liver injury is usually mild-to-moderate in severity and resolves rapidly with stopping. However, fatal instances have been reported.
Likelihood score: B (highly likely cause of clinically apparent liver injury).
Mechanism of Injury
The cause of ALT elevations during high dose clindamycin therapy is not known, but may be due to a direct but mild injury to the liver. Liver biopsy during these episodes generally shows minimal cell injury. The idiosyncratic reaction to clindamycin resembles the immunoallergic types of hepatitis that occur after many types of antibiotics, including the penicillins and cephalosporins.
Outcome and Management
The serum aminotransferase elevations that appear during high dose intravenous therapy with clindamycin are usually benign, minimally symptomatic and resolve rapidly with stopping therapy or switching to oral forms of clindamycin. Acute idiosyncratic hepatitis can occur with either the parenteral or oral forms of clindamycin, but is rare. The liver injury can be symptomatic and prolonged and has been linked to one case of vanishing bile duct syndrome, but not with acute liver failure. Ordinarily, recovery can be expected in 4 to 8 weeks. Prednisone is not recommended. Patients should be told to avoid reexposure to clindamycin and other lincomycin derivatives.
Drug Class: Antiinfective Agents
CASE REPORTS
Case 1. Serum aminotransferase elevations during intravenous therapy with clindamycin.(1)
A 57 year old man with rheumatic heart disease and Staphylococcal endocarditis was treated with clindamycin (300 mg intravenously every 8 hours) and developed elevations in serum aminotransferase levels starting during the first week of therapy. Clindamycin was stopped at day 16 because of continuing elevations in ALT levels, which promptly began to fall when he was switched to cephalothin (Table). While blood cultures became negative on therapy, he subsequently died of E. coli sepsis.
Key Points
Laboratory Values
Comment
A typical pattern of serum aminotransferase elevations during intravenous clindamycin therapy that can be seen with several antibiotics, most typically with oxacillin. The liver injury usually arises after 4 to 6 days and is minimally symptomatic, anicteric and resolves rapidly when the antibiotic is stopped. The injury is probably dose related and a direct toxic effect of clindamycin or oxacillin.
Case 2. Acute hepatitis arising during intravenous therapy with clindamycin.(2)
An 18 year old man with acute bacterial endocarditis related to injection drug use developed elevated serum aminotransferases and then jaundice during intravenous clindamycin therapy. The patient had been treated with high doses of cephalothin and vancomycin for over two months with an inadequate response, during which time his liver tests were normal. Blood cultures revealed Staphylococcus aureus sensitive to clindamycin, and he was started on intravenous clindamycin (3.6 g/day) with a rapid clinical improvement. After a week, however, serum AST levels began to rise. The dose of clindamycin was cut in half, but he subsequently developed urticaria, recurrence of fever, jaundice and rising AST levels (Table). Tests for hepatitis B were negative, and liver biopsy showed acute hepatitis without fibrosis compatible with a drug induced liver injury. Clindamycin was stopped and the rash, fever and jaundice promptly resolved. Follow up blood cultures were negative and serum AST levels were normal when tested one month later.
Key Points
Laboratory Values
| Time After Starting | Time After Stopping | AST* (U/L) | Alk P* (U/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|---|
| Clindamycin (3.6 gm IV daily) started for endocarditis | |||||
| Pre | 30 | 59 | 0.8 | ||
| 5 days | 40 | ||||
| 8 days | 110 | ||||
| 10 days | 310 | ||||
| 14 days | 480 | 1.5 | |||
| 16 days | 710 | Reduced dose | |||
| 3 weeks | 750 | 80 | 3.5 | ||
| 4 weeks | 80 | 1.8 | Liver biopsy | ||
| Fever and urticaria: clindamycin stopped | |||||
| 5 weeks | 4 days | 50 | |||
| 6 weeks | 10 days | 80 | 1.1 | ||
| 7 weeks | 18 days | 160 | Normal | ||
| 2 months | 1 month | 30 | 0.9 | ||
| Normal Values | <40 | <40 | <1.2 | ||
- *
Values estimated from Figure 1.
Comment
A typical pattern of serum aminotransferase elevations arising after a week of intravenous clindamycin therapy. The clindamycin dose was decreased because of the rising AST levels, but fever and urticaria arose, and clindamycin was stopped, whereupon the liver injury resolved rapidly. This example suggests that the asymptomatic elevations in serum aminotransferase levels during intravenous clindamycin therapy can evolve into the immunoallergic and icteric form of liver injury if therapy is continued. This report predated the availability of tests for hepatitis C, which may have been at least partially responsible for the liver injury in this patient.
Case 3. Acute hepatitis with jaundice arising during clindamycin therapy.(3)
A 42 year old woman developed fatigue, nausea, anorexia, pruritus and dark urine followed by jaundice 6 days after starting a course of clindamycin (150 mg four times a day) for a gingival infection. She had received clindamycin for this same problem several times in the past without complications. She had no history of liver disease, alcohol abuse or risk factors for viral hepatitis and was taking no other medications. On presentation, she was jaundiced but without rash or fever. Laboratory testing showed an ALT of 1795 U/L, AST 1337 U/L, alkaline phosphatase 339 U/L, GGT 148 U/L, total bilirubin 4.1 mg/dL (direct 2.9 mg/dL) and INR 1.04. The eosinophil count was normal. Tests for hepatitis A, B and C and for cytomegalovirus and Epstein Barr virus infection were negative. Autoantibodies, including ANA, SMA and anti-LKM, were negative and an abdominal ultrasound showed no evidence of biliary obstruction. A liver biopsy showed cholestatic hepatitis without fibrosis, suggestive of drug induced liver injury. After 3 weeks, she was largely asymptomatic and all liver tests had returned to normal when she was seen 8 weeks after presentation (Table).
Key Points
| Medication: | Clindamycin, 150 mg 4 times daily |
|---|---|
| Pattern: | Hepatocellular (R=44.9) |
| Severity: | 3+ (jaundiced and hospitalized) |
| Latency: | 6 days to symptoms |
| Recovery: | Within two months |
| Other medications: | None |
Laboratory Values
Comment
While referred to as “cholestatic” in the title of the publication, the clinical presentation was clearly hepatocellular and acute viral hepatitis-like arising after a week of clindamycin therapy. No other medications were being taken and other causes of liver injury were adequately excluded. The liver injury was clearly idiosyncratic and likely due to hypersensitivity, despite the absence of immunoallergic features (rash, fever and eosinophilia). Previous courses of clindamycin may have sensitized the patient to the antibiotic. Isolated instances of cross sensitivity have been reported between clindamycin and the penicillins and future use of related antibiotics should be done with care.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Clindamycin – Generic, Cleocin®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Clindamycin | 18323-44-9 | C18-H33-Cl-N2-O5-S |
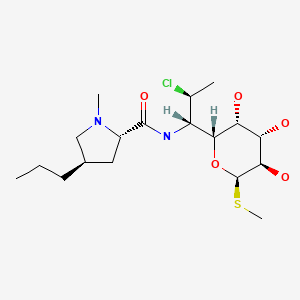
|
CITED REFERENCES
- 1.
- Modified from Case #3: Hinthorn DR, Baker LH, Romig DA, Voth DW, Liu C. Endocarditis treated with clindamycin: relapse and liver dysfunction. South Med J. 1977;70:823–6. [PubMed: 877644]
- 2.
- Elmore M, Rissing JP, Rink L, Brooks GF. Clindamycin-associated hepatotoxicity. Am J Med. 1974;57:627–30. [PubMed: 4432865]
- 3.
- Aygun C, Kocaman O, Gurbuz Y, Senturk O, Hulagu S. Clindamycin-induced acute cholestatic hepatitis. World J Gastroenterol. 2007;13:5408–10. [PMC free article: PMC4171338] [PubMed: 17879418]
ANNOTATED BIBLIOGRAPHY
References updated: 16 December 2019
- Zimmerman HJ. Clindamycin. Hepatic injury from antimicrobial agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, p. 592.(Expert review of liver injury published in 1999 mentions that ALT elevations are common during clindamycin therapy, and 2 cases of hepatocellular jaundice attributed to clindamycin have been published).
- Moseley RH. Clindamycin. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced Liver Disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 470.(Expert review of antibiotic induced liver injury mentions that clindamycin has been rarely reported to cause hepatotoxicity).
- MacDougall C. Lincosamides (Clindamycin). Protein synthesis inhibitors and miscellaneous antibacterial agents. In, Brunton LL, Hillal-Dandan R, Knollman BA, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1056-7.(Textbook of pharmacology and therapeutics).
- Elmore MF, Rink LD, Rissing JP. Clindamycin, endocarditis, hepatotoxicity. Ann Intern Med. 1973;78:779–80. [PubMed: 4711784](Letter to editor describing 18 year old injection drug user with acute bacterial endocarditis who developed marked AST elevation [710 U/L] after 18 days of iv clindamycin, resolving rapidly with stopping; 2 biopsies, first during therapy showed mild hepatitis, second during recovery showed resolution).
- Fass RJ, Scholand JF, Hodges GR, Saslaw S. Clindamycin in the treatment of serious anaerobic infections. Ann Intern Med. 1973;78:853–9. [PubMed: 4713566](Analysis of responses to parenteral clindamycin in 19 adults with serious infections, 8 of who developed mild, transient ALT and AST or Alk P elevations, but none required discontinuation or developed jaundice).
- Craig JA. Jaundice in acute pustular psoriasis. Br Med J. 1974;3:43. [PMC free article: PMC1611415] [PubMed: 4835475](62 year old woman developed jaundice 3 days after starting clindamycin therapy of sepsis [bilirubin 12.2 mg/dL, ALT normal]; likely due to sepsis rather than clindamycin).
- Elmore M, Rissing JP, Rink L, Brooks GF. Clindamycin-associated hepatotoxicity. Am J Med. 1974;57:627–30. [PubMed: 4432865](18 year old injection drug use with acute bacterial endocarditis developed jaundice [3.5 mg/dL] and AST elevations [710 U/L] after 21 days of iv clindamycin, resolving rapidly once therapy was stopped; two biopsies done, first during therapy showed mild hepatitis, second during recovery showed resolution: Case 2).
- Levison ME, Bran JL, Ries K. Treatment of anaerobic bacterial infections with clindamycin-2-phosphate. Antimicrob Agents Chemother. 1974;5:276–80. [PMC free article: PMC428960] [PubMed: 4600161](Among 35 patients treated with clindamycin im or iv for severe bacterial infections, mild elevations in ALT, AST and Alk P were common, one patient developed hepatitis 1 day after stopping 12 day course of clindamycin [bilirubin 5.6 mg/dL, ALT 680 U/L, Alk P 460 U/L], resolving within 3-4 months of stopping).
- Williams DN, Crossley K, Hoffman C, Sabath LD. Parenteral clindamycin phosphate: pharmacology with normal and abnormal liver function and effect on nasal staphylococci. Antimicrob Agents Chemother. 1975;7:153–8. [PMC free article: PMC429095] [PubMed: 1137366](Open label study of parenteral clindamycin therapy in 41 patients including 24 with preexisting liver disease; AST levels rose in 5 patients with liver disease and improved in others; among 17 patients without liver disease, AST levels rose in 4 [52, 57, 60 and 270 U/L], but elevations were self-limited and resolved spontaneously).
- Hinthorn DR, Baker LH, Romig DA, Voth DW, Liu C. Endocarditis treated with clindamycin: relapse and liver dysfunction. South Med J. 1977;70:823–6. [PubMed: 877644](Among 6 patients treated for endocarditis with low rate of response, 2 developed ALT [~200 and 550 U/L], AST and LDH elevations after 8-18 days without jaundice or symptoms, resolving within 2 weeks of discontinuation: Case 1).
- Kobayasi A, Sato S, Ito T, Utsumi T, Kikuchi N. Jpn J Antibiot. 1977;30:13–21. [Therapy for severe anaerobic infections with clindamycin (author's transl)] Japanese. [PubMed: 839638](From abstract: Among 11 patients given oral clindamycin for up to 49 days, one developed transient ALT elevations).
- Prospective, randomized comparison of metronidazole and clindamycin, each with gentamicin, for the treatment of serious intra-abdominal infection. Surgery. 1983;93(1 Pt 2):221–9. [PubMed: 6849209](Multicenter study comparing clindamycin to metronidazole in 186 patients with severe infections; similar efficacy, AST elevations in 18% vs 10%, but no frank hepatitis or discontinuation for liver injury).
- Altraif I, Lilly L, Wanless IR, Heathcote J. Cholestatic liver disease with ductopenia (vanishing bile duct syndrome) after administration of clindamycin and trimethoprim-sulfamethoxazole. Am J Gastroenterol. 1994;89:1230–4. [PubMed: 8053440](Two cases of vanishing bile duct syndrome, one due to clindamycin: a 67 year old man with jaundice arising 1 week after completion of a 10 day course [bilirubin 14.6 mg/dL, ALT 315 U/L, Alk P 271 U/L, GGT 468 U/L], followed by prolonged jaundice for 2-4 years [bilirubin 4.2 mg/dL, Alk P 179 U/L, ALT 67 U/L], liver biopsies showing progressive loss of bile ducts; ampicillin caused sudden worsening).
- Maraqa NF, Gomez MM, Rathore MH, Alvarez AM. Higher occurrence of hepatotoxicity and rash in patients treated with oxacillin, compared with those treated with nafcillin and other commonly used antimicrobials. Clin Infect Dis. 2002;34:50–4. [PubMed: 11731945](Retrospective review of 222 children receiving outpatient parenteral antibiotics; 22% receiving oxacillin, but only 1 of 72 receiving clindamycin developed “hepatitis” [at 17 days: bilirubin 0.6 mg/dL, ALT 183 U/L, AP normal]).
- De Valle MB, Av Klinteberg V, Alem N, Olsson R, Björnsson E. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment Pharmacol Ther. 2006;24:1187–95. [PubMed: 17014577](Survey of 77 cases of drug induced liver injury seen over 10 years, found 2 cases of clindamycin related injury, both hepatocellular, with onset after 7 and 35 days, resolution in 2 weeks and 6 months).
- Aygun C, Kocaman O, Gurbuz Y, Senturk O, Hulagu S. Clindamycin-induced acute cholestatic hepatitis. World J Gastroenterol. 2007;13:5408–10. [PMC free article: PMC4171338] [PubMed: 17879418](42 year old woman developed jaundice and pruritus after 6 days of oral clindamycin [bilirubin 4.1 mg/dL, ALT 1795 U/L, Alk P 339 U/L], resolving within 8 weeks of stopping).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, clindamycin was implicated in one case).
- Flores Sahagun JE, Ortiz Soto JA, Mendez T, Cardenas Ochoa EC, Hernandez Flores G. Dermatology Online J. 2009;15:12. [Stevens Johsnon Syndrome and clindamycin-or chlorpheniramine-induced intrahepatic cholestatis] [PubMed: 19624990](48 year old woman developed jaundice at the end of an 8 day course of clindamycin and chlorpheniramine [bilirubin 3.5 mg/dL, ALT 1105 U/L, Alk P 444 U/L], with an accompanying rash that spread to mouth, palms and soles, responding to corticosteroids with rapid recovery within weeks).
- Leitner JM, Graninger W, Thalhammer F. Hepatotoxicity of antibacterials: Pathomechanisms and clinical. Infection. 2010;38:3–11. [PubMed: 20107858](Review of frequency and cause of antibiotic induced liver injury, rates range from 0.2 [moxifloxacin] to 17 [amoxicillin/ clavulanate] per 100,000 prescriptions for different commonly implicated agents; mentions that clindamycin most frequently causes asymptomatic increases in ALT).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 31 due to antibiotics, but none were attributed to clindamycin).
- Bawany MZ, Bhutto B, Youssef WI, Nawras A, Sodeman T. Acute liver failure: an uncommon complication of commonly used medication. Am J Ther. 2011;20:566–8. [PubMed: 21642826](73 year old man developed jaundice 7 days after starting clindamycin [bilirubin 6.9 mg/dL, ALT 1076 U/L, Alk P 203 U/L, INR 2.1], liver tests improved on stopping, but patient developed respiratory followed by multiorgan failure and died soon thereafter).
- Kwon H, Lee SH, Kim SE, Lee JH, Jee YK, Kang HR, Park BJ, et al. Spontaneously reported hepatic adverse drug events in Korea: multicenter study. J Korean Med Sci. 2012;27:268–73. [PMC free article: PMC3286773] [PubMed: 22379337](Summary of 2 years of adverse event reporting in Korea; of 9360 reports, 567 were liver related, including 6 [1.1%] attributed to clindamycin).
- Miller Quidley A, Bookstaver PB, Gainey AB, Gainey MD. Fatal clindamycin-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Pharmacotherapy. 2012;32:e387–92. [PubMed: 23165860](63 year old woman developed nausea and a generalized erythematous rash 4 days after starting a course of clindamycin, with subsequent fever, eosinophilia [24%], lymphadenopathy, renal dysfunction, pancreatitis, and liver injury [bilirubin 4.3 mg/dL, ALT 67 U/L, Alk P 1010 U/L], with progressive multiorgan failure and death 16 days after presentation).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;114:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, of which none were attributed to clindamycin).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, including 39 attributed to antimicrobial agents, but none to clindamycin).
- Lim R, Choudry H, Conner K, Karnsakul W. A challenge for diagnosing acute liver injury with concomitant/sequential exposure to multiple drugs: can causality assessment scales be utilized to identify the offending drug? Case Rep Pediatr. 2014;2014:156389. [PMC free article: PMC4260426] [PubMed: 25506455](12 year old boy with severe cellulitis received amoxicillin, ceftriaxone, vanocmycin, ampicillin/sulbactam and clindamycin, whereupon he developed fever and jaundice [bilirubin 3.0 rising to 11.8 mg/dL, ALT 406 U/L, Alk P 404 U/L], resolving slowly and incompletely by 6 months; causality was complicated by polypharmacy, but authors attributed the injury to clindamycin).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 [36%] were attributed to antibiotics including two due to clindamycin; both with jaundice [initial bilirubin 3.4 and 6.4 mg/dL], one being hepatocellular, one mixed, both self limited).
- Moole H, Ahmed Z, Saxena N, Puli SR, Dhillon S. Oral clindamycin causing acute cholestatic hepatitis without ductopenia: a brief review of idiosyncratic drug-induced liver injury and a case report. J Community Hosp Intern Med Perspect. 2015;5:28746. [PMC free article: PMC4612703] [PubMed: 26486111](75 year old woman developed jaundice while receiving a 10 day course of clindamycin [bilirubin 9.2 rising to 25 mg/dL, ALT 227 U/L, Alk P 296 U/L, INR 1.5 rising to 2.5], resolving within 4 weeks on prednisone therapy).
- Okudo J, Anusim N. Hepatotoxicity due to clindamycin in combination with acetaminophen in a 62-year-old African American female: a case report and review of the literature. Case Reports Hepatol. 2016;2016:2724738. [PMC free article: PMC4947643] [PubMed: 27462474](62 year old woman developed liver test abnormalities 5 days after starting clindamycin [bilirubin normal, ALT 423, Alk P 321 U/L] and rose while it was continued [peak ALT 1927 U/L], with improvement on stopping).
- Munz M, Grummich H, Birkmann J, Wilhelm M, Holzgrabe U, Sörgel F. Severe drug-induced liver injury as an adverse drug event of antibiotics: a case report and review of the literature. Chemotherapy. 2017;62:367–73. [PubMed: 28934748](20 year old woman developed jaundice having recently received courses of amoxicillin, cefazolin and clindamycin [bilirubin 3.7 rising to 16.9 mg/dL, ALT 1219 U/L, Alk P 143 U/L, INR 1.57 rising to 2.6], with progressive liver failure but eventual spontaneous recovery with normal liver tests 2 months later).
- Hoofnagle JH, Björnsson ES. Drug-induced liver injury - types and phenotypes. N Engl J Med. 2019;381:264–73. [PubMed: 31314970](Review of the clinical features, diagnosis, complications and pathogenesis of different forms of drug induced liver injury mentions that antibiotics are the most common medications causing liver injury, but they are also the most common class of medications approved for use in the US).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Tobramycin.[LiverTox: Clinical and Researc...]Review Tobramycin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Oral clindamycin causing acute cholestatic hepatitis without ductopenia: a brief review of idiosyncratic drug-induced liver injury and a case report.[J Community Hosp Intern Med Pe...]Oral clindamycin causing acute cholestatic hepatitis without ductopenia: a brief review of idiosyncratic drug-induced liver injury and a case report.Moole H, Ahmed Z, Saxena N, Puli SR, Dhillon S. J Community Hosp Intern Med Perspect. 2015; 5(5):28746. Epub 2015 Oct 19.
- Use of antibiotics in dental practice.[Dent Clin North Am. 1984]Use of antibiotics in dental practice.Montgomery EH, Kroeger DC. Dent Clin North Am. 1984 Jul; 28(3):433-53.
- Antibiotic treatment of tuboovarian abscess: comparison of broad-spectrum beta-lactam agents versus clindamycin-containing regimens.[Am J Obstet Gynecol. 1991]Antibiotic treatment of tuboovarian abscess: comparison of broad-spectrum beta-lactam agents versus clindamycin-containing regimens.Reed SD, Landers DV, Sweet RL. Am J Obstet Gynecol. 1991 Jun; 164(6 Pt 1):1556-61; discussion 1561-2.
- Review Amikacin.[LiverTox: Clinical and Researc...]Review Amikacin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Clindamycin - LiverToxClindamycin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...