NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Clobazam is a benzodiazepine that is used as an anticonvulsant in the therapy of severe childhood epilepsy. Therapy with clobazam has not been associated with serum aminotransferase elevations, and clinically apparent liver injury from clobazam has yet to be reported and must be rare, if it occurs at all.
Background
Clobazam (kloe' ba zam) is a unique 1,5 benzodiazepine which is used in the United States as an anticonvulsant, but is available in other countries for therapy of anxiety as well as epilepsy. The anticonvulsant activity of clobazam appears to be mediated by its ability to enhance gamma-aminobutyric acid (GABA) medicated inhibition of synaptic transmission via binding to the GABA-A receptor. Clobazam was approved for use in the United States in 2011 as an anticonvulsant to be used either alone or in combination with other agents as therapy of the severe form of childhood epilepsy known as Lennox-Gastaut syndrome. Clobazam is available in tablets of 5, 10 and 20 mg under the brand name Onfi in the United States and Frisium and Urbanol elsewhere. The recommended initial dose is 5 mg once or twice daily, with subsequent increases to a usual maintenance dose of 20 mg twice daily. The dose should be increased and tapered gradually. The most common side effects are dose related and include dizziness, somnolence, impaired concentration, nervousness, aggression, antegrade amnesia, headache, fatigue, nausea and weakness. Clobazam can also lead to physical dependence and sudden discontinuation can cause withdrawal symptoms and rebound seizure activity.
Hepatotoxicity
Limited data are available on the hepatotoxicity of clobazam. In clinical trials, clobazam was not associated with an increased frequency of serum aminotransferase elevations as compared to placebo treatment, and there were no instances of clinically apparent liver injury. No individual case reports of clobazam hepatotoxicity have been published since its wide spread clinical availability. Liver injury from benzodiazepines is quite rare, but isolated instances of hepatotoxicity have been reported for clorazepate and clonazepam, two other benzodiazepines used predominantly in the therapy of epilepsy. Thus, clinically apparent liver injury due to clobazam must be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Clobazam is extensively metabolized by the liver, largely by glucuronidation and acetylation. Its metabolism is independent of the microsomal P450 system, and it has little or no effect on P450 activity and lacks significant drug-drug interactions.
Outcome and Management
There is no information on the possible cross sensitivity to hepatotoxicity among the various benzodiazepines used as anticonvulsants such as diazepam, clonazepam, clorazepate or clobazam. Nevertheless, switching from one to another after clinically apparent liver injury should be done with caution and with appropriate clinical monitoring.
Drug Class: Anticonvulsants, Benzodiazepines
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Clobazam – Onfi®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Clobazam | 22316-47-8 | C16-H13-Cl-N2-O2 |
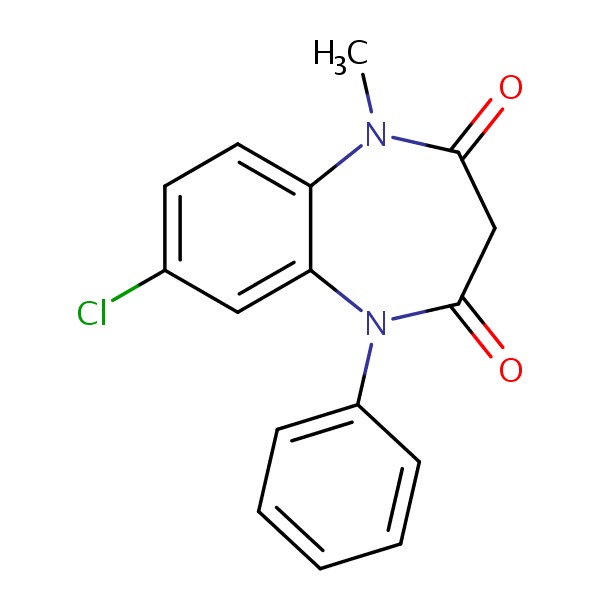 |
| Clonazepam | 1622-61-3 | C15-H10-Cl-N3-O3 |
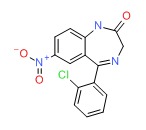 |
| Clorazepate | 57109-90-7 | C16-H10-Cl-N4-O3.K.H-K-O |
 |
| Diazepam | 439-14-5 | C16-H13-Cl-N2-O |
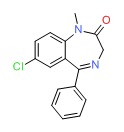 |
ANNOTATED BIBLIOGRAPHY
References updated: 25 January 2017
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999; diazepam, but not clobazam is discussed).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-42.(Review of anticonvulsant induced liver injury; clobazam is not discussed).
- McNamara JO. Pharmacology of the epilepsies. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 583-607.(Textbook of pharmacology and therapeutics).
- Jacobson AF, Goldstein BJ, Dominguez RA, Steinbook RM. A placebo-controlled, double-blind comparison of clobazam and diazepam in the treatment of anxiety. J Clin Psychiatry 1983; 44: 296-300. [PubMed: 6135690](Controlled trial of 4 weeks of diazepam vs clobazam vs placebo in 114 patients with anxiety found sedation similar with either drug, but dizziness more frequent with diazepam; no mention of ALT elevations or hepatotoxicity, although ALT was measured at the start and end of therapy).
- Clobazam in treatment of refractory epilepsy: the Canadian experience. A retrospective study. Canadian Clobazam Cooperative Group. Epilepsia 1991; 32: 407-16. [PubMed: 2044502](Retrospective survey of Canadian neurologists with experience using clobazam in refractory epilepsy in a total of 877 patients; 32% had adverse events, most commonly somnolence, dizziness, ataxia, mood changes, hostility, blurred vision and weight gain; no mention of ALT elevations or hepatotoxicity).
- Conry JA, Ng YT, Paolicchi JM, Kernitsky L, Mitchell WG, Ritter FJ, Collins SD, Tracy K, et al. Clobazam in the treatment of Lennox-Gastaut syndrome. Epilepsia 2009; 50: 1158-66. [PubMed: 19170737](Among 68 children with Lennox-Gastaut syndrome who were treated with either low or high dose clobazam for 7 weeks, seizure reduction was more frequent with the higher dose as were side effects, which included somnolence, sedation, constipation, salivation, insomnia, irritability and lability; no mention of ALT elevations or hepatotoxicity).
- Ng YT, Conry JA, Drummond R, Stolle J, Weinberg MA; OV-1012 Study Investigators. Randomized, phase III study results of clobazam in Lennox-Gastaut syndrome. Neurology. 2011; 77: 1473-81. [PubMed: 21956725](Among 238 patients with Lennox-Gastaut syndrome treated with one of 3 doses of clobazam or placebo for at least 18 weeks, no patient developed serious hepatic adverse events and there were no abnormal clinical laboratory results linked to clobazam therapy).
- Clobazam (Onfi) for Lennox-Gastaut syndrome. Med Lett Drugs Ther 2012; 54 (1385): 18-9. [PubMed: 22382580](Concise review of the pharmacology, clinical efficacy, adverse events, drug interactions and costs of clobazam for Lennox-Gastaut syndrome shortly after its approval for this indication in the US; no mention of ALT elevations or hepatotoxicity).
- Yang LP, Scott LJ. Clobazam : in patients with Lennox-Gastaut syndrome. CNS Drugs 2012; 26: 983-91. [PubMed: 23034582](Summary of the structure, mechanism of action, pharmacology, clinical efficacy and safety of clobazam for Lennox Gastaut syndrome; no mention of hepatotoxicity or ALT elevations).
- Ng YT, Conry J, Paolicchi J, Kernitsky L, Mitchell W, Drummond R, Isojarvi J, Lee D, Owen R; OV-1004 study investigators. Long-term safety and efficacy of clobazam for Lennox-Gastaut syndrome: interim results of an open-label extension study. Epilepsy Behav 2012; 25: 687-94. [PubMed: 23141144](Among 267 children and adults who participated in controlled trials of clobazam and were enrolled in a long term extension study, 13 discontinued therapy because of side effects and six died, but none were attributed to hepatotoxicity).
- Wheless JW, Phelps SJ. Clobazam: a newly approved but well-established drug for the treatment of intractable epilepsy syndromes. J Child Neurol 2013; 28: 219-29. [PubMed: 23112237](Review of the pharmacology, safety and efficacy of clobazam in Lennox Gastaut syndrome; no mention of hepatotoxicity).
- Drugs for epilepsy. Treat Guidel Med Lett 2013; 11: 9-18. [PubMed: 23348233](Concise review of indications and side effects of anticonvulsants; clobazam is approved only for treatment of severe seizures associated with Lennox-Gastaut syndrome; no mention of hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the 96, none were attributed to clobazam or other benzodiazepines).
- Klehm J, Thome-Souza S, Sánchez Fernández I, Bergin AM, Bolton J, Harini C, Kadish NE, et al. Clobazam: effect on frequency of seizures and safety profile in different subgroups of children with epilepsy. Pediatr Neurol 2014; 51: 60-6. [PubMed: 24830765](Among 300 children with seizures treated with clobazam for more than 1 month at a single referral center, 28% became seizure free and the most common side effects were tiredness [15%] and mood or behavioral changes [8%]; no mention of ALT elevations or hepatotoxicity).
- Conry JA, Ng YT, Kernitsky L, Mitchell WG, Veidemanis R, Drummond R, Isojarvi J, Lee D, et al.; OV-1004 Study Investigators. Stable dosages of clobazam for Lennox-Gastaut syndrome are associated with sustained drop-seizure and total-seizure improvements over 3 years. Epilepsia 2014; 55: 558-67. [PMC free article: PMC4303987] [PubMed: 24580023](Among 267 patients with Lennox-Gastaut syndrome enrolled in a long term, open label, observational study of clozabam, adverse events were similar to those in controlled trials and none of the serious adverse events or the 18 discontinuations because of side effects or the 10 deaths were attributed to liver injury).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were attributed to anticonvulsants, but none to clobazam or other benzodiazepine anticonvulsants).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review GABA(A) receptor physiology and its relationship to the mechanism of action of the 1,5-benzodiazepine clobazam.[CNS Drugs. 2012]Review GABA(A) receptor physiology and its relationship to the mechanism of action of the 1,5-benzodiazepine clobazam.Sankar R. CNS Drugs. 2012 Mar 1; 26(3):229-44.
- Anticonvulsant action of a 1,5-benzodiazepine, clobazam, in reflex epilepsy.[Epilepsia. 1978]Anticonvulsant action of a 1,5-benzodiazepine, clobazam, in reflex epilepsy.Chapman AG, Horton RW, Meldrum BS. Epilepsia. 1978 Jun; 19(3):293-9.
- N-desmethylclobazam: a possible alternative to clobazam in the treatment of refractory epilepsy?[Br J Clin Pharmacol. 1987]N-desmethylclobazam: a possible alternative to clobazam in the treatment of refractory epilepsy?Haigh JR, Pullar T, Gent JP, Dailley C, Feely M. Br J Clin Pharmacol. 1987 Feb; 23(2):213-8.
- Clobazam in the treatment of epilepsy.[Clin Exp Neurol. 1987]Clobazam in the treatment of epilepsy.Kilpatrick C, Bury R, Fullinfaw R, Moulds R. Clin Exp Neurol. 1987; 23:139-44.
- Review Clobazam: A Safe, Efficacious, and Newly Rediscovered Therapeutic for Epilepsy.[CNS Neurosci Ther. 2015]Review Clobazam: A Safe, Efficacious, and Newly Rediscovered Therapeutic for Epilepsy.Gauthier AC, Mattson RH. CNS Neurosci Ther. 2015 Jul; 21(7):543-8. Epub 2015 Apr 28.
- Clobazam - LiverToxClobazam - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...