NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Octreotide is a synthetic somatostatin analogue that resembles the native polypeptide in its activity in suppressing levels and activity of growth hormone, insulin, glucagon and many other gastrointestinal peptides. Because its half-life is longer than somatostatin, octreotide can be used clinically to treat neuroendocrine tumors that secrete excessive amounts of growth hormone (acromegaly) or other active hormones or neuropeptides. Octreotide has many side effects including suppression of gall bladder contractility and bile production, and maintenance therapy can cause cholelithiasis, pancreatitis as well as clinically apparent liver injury.
Background
Octreotide (ok tree' oh tide) is a synthetic cyclic octapeptide and analogue of somatostatin that is used for its ability to suppress levels and activities of other hormones (growth hormone, insulin, gastrin, secretin, glucagon) or active neuropeptides (serotonin, vasoactive intestinal polypeptide [VIP]). Natural somatostatin is produced in the hypothalamus and acts to suppress growth hormone release from the pituitary. Somatostatin is also found in other neurons throughout the body and particularly in intestinal and pancreatic neurons, where it is active in suppressing release of other hormones and neuropeptides such as insulin, glucagon, gastrin, secretin, motilin, VIP, serotonin and cholecystokinin. Octreotide has a longer half-life (1 to 2 hours) than somatostatin (~3 minutes) and yields effective suppression of hormone production when given three times daily. More recently, longer acting formulations of octreotide have been developed that can be administered weekly or monthly. Octreotide appears to interact largely with the somatostatin subtype 2 and possibly subtype 5 receptors, with little effect on subtypes 1, 3 and 4, explaining its focused effects. Octreotide therapy has been shown to improve symptoms and complications of several neuroendocrine tumors including abnormal growth in acromegaly due to growth hormone secreting pituitary tumors, diarrhea due to VIP secreting intestinal tumors and flushing due to carcinoid tumors. Octreotide was approved for use in the United States in 1988 and current listed indications include acromegaly, watery diarrhea from VIP producing tumors and diarrhea and flushing due to metastatic carcinoid tumors. Octreotide has been used off-label for portal hypertension to control variceal hemorrhage as well as for dumping syndrome and other gastrointestinal motility disorders. Octreotide is available generically and under the brand name Sandostatin in several forms for intravenous or subcutaneous administration. A long acting form is available that can be given monthly (Sandostatin LAR Depot). The typical dose in adults is 100 mcg given as injections three times daily in 2 weeks courses, and then for long term maintenace as injections 2 to 4 times daily or less frequently with long acting formulations. Side effects are common. Adverse events from single injections include influenza-like symptoms of fatigue, headache, nausea and vomiting and local infusion reactions. With continued therapy, adverse events can include diarrhea, abdominal pain, back pain, headache, dizziness, hypothyroidism, hypo- and hyperglycemia, arrhythmias and gall bladder disease.
Hepatotoxicity
Mild, transient, asymptomatic elevations in serum aminotransferase levels occur in a small proportion of patients receiving octreotide, and in some individuals the elevations are persistent and worsen over time and may require drug discontinuation. In addition, several instances of acute, clinically apparent liver injury attributable to octreotide have been described. The onset is generally within 1 to 6 months of starting therapy and injury may be more frequent with higher doses. Most cases of liver injury associated with octreotide therapy have been asymptomatic and anicteric, and marked by prominent elevations in serum ALT and AST with normal or near normal serum alkaline phosphatase, GGT and bilirubin. In some instances, however, jaundice has arisen, particularly with rechallenge. There have been no instances of acute liver failure or vanishing bile duct syndrome associated with octreotide, and a characteristic feature of the injury is the rapidity of improvement upon stopping the injections or infusions. Several instances of marked aminotransferase elevations with rapid improvements on stopping have been reported in newborns and infants with congenital hyperinsulinemia who were treated with continuous infusions of high doses of octreotide.
Octreotide causes inhibition of gall bladder contractility and decrease in bile secretion, and long term therapy is associated with a high rate of cholesterol gallstone formation. In prospective studies, between 25% and 65% of patients with acromegaly treated with maintenance octreotide developed gallstones detected by ultrasonography and a proportion developed symptomatic cholelithiasis requiring hospitalization and cholecystectomy. Even after cholecystectomy, cholesterol stones may form in the common bile duct and intrahepatic ducts causing symptoms, episodes of sepsis and need for partial hepatic resection. Therapy with ursodiol does not appear to prevent gallstone formation during octreotide therapy, although it may help. Octreotide has also been associated with acute pancreatitis, which may be due to its inhibitory effect on gastrointestinal hormone release, although other cases may be secondary to passage of gall bladder stones and pancreatic duct obstruction.
Likelihood score: C (probable cause of clinically apparent liver injury).
Mechanism of Injury
Octreotide, like somatostatin, decreases cholecystokinin secretion, gall bladder contractility and bile secretion, perhaps accounting for the high rate of gall bladder sludge and stone formation with long term use. How infusions of octreotide cause acute liver injury independent of its effect on bile flow and gall bladder function is uncertain. Octreotide is a polypeptide and, as such, should not have direct or even indirect hepatic toxicity. Thus, the liver injury, like the gall bladder effects, is likely caused by what octreotide does, rather than by its chemical structure or what it is. Octreotide has multiple effects on gastrointestinal motility and changes bowel transient, bacterial composition in the intestines and bile acid levels. In addition, glycogenosis caused by changes in insulin and glucagon is a possible cause of the serum ALT and AST elevations that occur with high doses of octreotide and would account for the rapidity of improvement upon stopping.
Outcome and Management
The liver injury due to the octreotide generally resolves rapidly with stopping therapy. It also usually recurs upon restarting and may be more severe. For these reasons, patients that are restarted on treatment after an episode of liver injury should be monitored carefully for possible recurrence.
Drug Class: Hormonal Agents; Antineoplastic Agents, Peptide Hormones; Gastrointestinal Agents, Antidiarrheals
Other Drugs in the Subclass, Hormonal Agents, Somatostatin Analogues: Lanreotide, Pasireotide
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Octreotide – Generic, Sandostatin®
DRUG CLASS
Hormonal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lanreotide | 108736-35-2 | C54-H69-N11-O10-S2 |
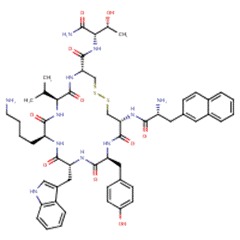 |
| Octreotide | 83150-76-9 | C49-H66-N10-O10-S2 |
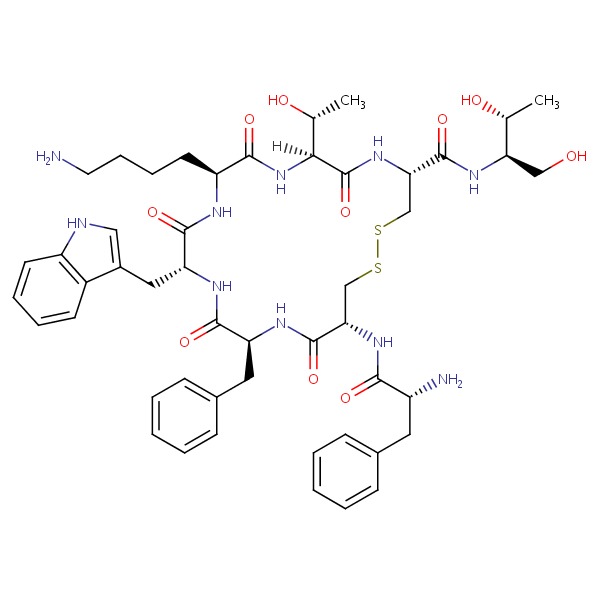 |
| Pasireotide | 396091-73-9 | C58-H66-N10-O9 |
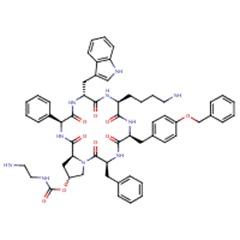 |
| Somatostatin | 51110-01-1 | Protein | Not Available |
ANNOTATED BIBLIOGRAPHY
References updated: 30 March 2016
- Zimmerman HJ. Hepatic injury from the treatment of infectious and parasitic diseases. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 589-637.(Expert review of hepatotoxicity published in 1999, mentions that octreotide has been implicated in several cases of acute hepatocellular injury which subsided after drug withdrawal).
- Kaplowitz N, DeLeve LD, eds. Drug-induced Liver Disease. 3rd edition. Amsterdam: Elsevier, 2013.(Multiauthored text book on drug induced liver injury; does not discuss octreotide).
- Sharkey KA, Wallace JL. Treatment of disorders of bowel motility and water flux: anti-emetics; agents used in biliary and pancreatic disease. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1323-49.(Textbook of pharmacology and therapeutics).
- Kvols LK, Moertel CG, O'Connell MJ, Schutt AJ, Rubin J, Hahn RG. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med 1986; 315: 663-6. [PubMed: 2427948](Among 25 patients with metastatic carcinoid tumors treated with octreotide for 2 to 18 months, tolerance was “excellent” and “no evidence of …hepatic... toxicity was found”).
- Arosio M, Bazzoni N, Ambrosi B, Faglia G. Acute hepatitis after treatment of acromegaly with octreotide. Lancet 1988; 2 (8626-8627): 1498. [PubMed: 2904619](50 year old man with acromegaly developed abnormal liver tests without symptoms 5 months after starting octreotide [ALT 143 U/L, Alk P 1269 U/L, bilirubin not given], which resolved within 3 weeks of stopping and arose again with jaundice 3 months after restarting [bilirubin 12.8 mg/dL, ALT 2140 U/L, Alk P 1324 U/L], resolving again within 2 months of stopping).
- Ho KY, Weissberger AJ, Marbach P, Lazarus L. Therapeutic efficacy of the somatostatin analog SMS 201-995 (octreotide) in acromegaly. Effects of dose and frequency and long-term safety. Ann Intern Med 1990; 112: 173-81. [PubMed: 2404445](Among 18 patients with acromegaly treated with octreotide for at least one year, 9 [50%] developed gallstones, which were “small and numerous” and 2 [11%] developed symptoms and underwent cholecystectomy).
- Daughaday WH. Octreotide is effective in acromegaly but often results in cholelithiasis. Ann Intern Med 1990; 112: 159-60. [PubMed: 2404444](Editorial in response to Ho [1990] on the safety of octreotide in acromegaly recommends that it be limited to cases refractory to surgery).
- Minocha A, Dean HA Jr. Octreotide-induced acute hepatic toxicity. Am J Gastroenterol 1991; 86: 525. [PubMed: 2012056](53 year old man with cirrhosis and variceal hemorrhage developed serum enzyme elevations within 12 hours of starting infusions of octreotide [bilirubin levels not given, ALT rising from 59 to 1190 U/L, Alk P 81-151 U/L], enzymes decreasing rapidly upon stopping).
- Catnach SM, Anderson JV, Fairclough PD, Trembath RC, Wilson PA, Parker E, Besser GM, Wass JA. Effect of octreotide on gall stone prevalence and gall bladder motility in acromegaly. Gut 1993; 34: 270-3. [PMC free article: PMC1373983] [PubMed: 8432484](Gallstones were found by ultrasound in 34% of 39 acromegalic patients who had received octreotide vs 16% of 38 patients who had not).
- Hussaini SH, Pereira SP, Murphy GM, Kennedy C, Wass JA, Besser GM, Dowling RH. Composition of gall bladder stones associated with octreotide: response to oral ursodeoxycholic acid. Gut 1995; 36: 126-32. [PMC free article: PMC1382366] [PubMed: 7890216](Among 14 patients with acromegaly who were treated with octreotide and had gall bladder stones, chemical analysis in 2 showed cholesterol-rich stones; gall bladder bile in 6 was supersaturated with cholesterol; and ursodiol therapy resulted in partial or complete stone dissolution in 5 of 9 subjects).
- González-Martín JA, Donnay S, Morillas J, Gómez-Aparicio C, Garcia-Cano J, Pérez-Vigara G, Roldán A. Acute liver injury and octreotide. Am J Gastroenterol 1996; 91: 2434-5. [PubMed: 8931436](54 year old woman with acromegaly developed marked ALT elevations within a day of starting octreotide [ALT 538 U/L, GGT 133 U/L, bilirubin normal], which resolved within 2 weeks of stopping and recurred on rechallenge with a single dose [ALT 673 U/L]).
- Radetti G, Gentili L, Paganini C, Messner H. Cholelithiasis in a newborn following treatment with the somatostatin analogue octreotide. Eur J Pediatr 2000; 159: 550. [PubMed: 10923237](Newborn with congenital hyperinsulinemia developed jaundice 5 weeks after starting octreotide [bilirubin direct 3.6 and total 3.9 mg/dL, ALT 152 U/L, GGT 187 U/L], ultrasound showed stones in gall bladder and common duct, and abnormalities resolved within 6 weeks of lowering the octreotide dose and starting ursodiol).
- Sheehan MT, Nippoldt TB. Hepatolithiasis (intrahepatic stone) during octreotide therapy for acromegaly: a case report. Pituitary 2000; 3: 227-30. [PubMed: 11788010](27 year old man with acromegaly developed symptomatic cholelithiasis 5 years after starting octreotide and 2 years after cholecystectomyand presented later with recurrent painful intrahepatic stone disease [bilirubin 4.1 mg/dL, ALT 614 U/L, Alk P 192 U/L], ultimately requiring surgery).
- Crook MA, Steger A. Abnormal liver function tests in a patient fed with total parenteral nutrition and treated with octreotide. Nutrition 2001; 17: 152-4. [PubMed: 11240342](54 year old woman with complications after cholecystectomy developed modest rises in liver tests on octreotide which worsened on parenteral nutrition [peak bilirubin 2.1 mg/dL, ALT 183 U/L, Alk P 199 U/L], resolving completely only when both were stopped).
- Thomas LA, Veysey MJ, Murphy GM, Russell-Jones D, French GL, Wass JA, Dowling RH. Octreotide induced prolongation of colonic transit increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly. Gut 2005; 54: 630-5. [PMC free article: PMC1774470] [PubMed: 15831907](Octreotide caused increases in colonic transit times which affected deoxycholic acid levels in stool and serum, changes which might result in an increased risk for gallstones).
- Feenstra J, van Aken MO, de Herder WW, Feelders RA, van der Lely AJ. Drug-induced hepatitis in an acromegalic patient during combined treatment with pegvisomant and octreotide long-acting repeatable attributed to the use of pegvisomant. Eur J Endocrinol 2006; 154: 805-6. [PubMed: 16728538](45 year old man with acromegaly developed abnormal liver tests 5 months after pegvisomant [growth hormone receptor antagonist] was added to octreotide therapy [ALT 1148 U/L, Alk P 149 U/L, bilirubin not given], which decreased when pegvisomant was stopped and recurred when it was restarted alone without octreotide).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were due to octreotide).
- Avatapalle B, Padidela R, Randell T, Banerjee I. Drug-induced hepatitis following use of octreotide for long-term treatment of congenital hyperinsulinism. BMJ Case Rep 2012; 2012. [PMC free article: PMC4543377] [PubMed: 22850563](Baby girl with refractory congenital hyperinsulinemia developed rising ALT on high doses of octreotide [peak ALT 1061 U/L with normal Alk P and bilirubin], with “normal” liver biopsy and rapid resolution on stopping octreotide).
- Ben-Ari J, Greenberg M, Nemet D, Edelstein E, Eliakim A. Octreotide-induced hepatitis in a child with persistent hyperinsulinemia hypoglycemia of infancy. J Pediatr Endocrinol Metab 2013; 26: 179-82. [PubMed: 23327813](8 month old boy with congenital hyperinsulinemia developed liver test abnormalities on high doses of octreotide [ALT 535 U/L, AST 1065 U/L, with normal Alk P and bilirubin], liver biopsy showing portal inflammation and “hydropic degeneration”, resolving within 4 days of stopping).
- Koren I, Riskin A, Barthlen W, Gillis D. Hepatitis in an infant treated with octreotide for congenital hyperinsulinism. J Pediatr Endocrinol Metab 2013; 26: 183-5. [PubMed: 23327817](9 month old girl with congenital hyperinsulinemia developed abnormal liver tests 4 months after starting high doses of octreotide [peak ALT >1000 U/L with minimal increases in Alk P and GGT and normal bilirubin], falling to normal within 1 month of stopping).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, the most common implicated agents being nimesulide [n=53: 30%], cyproterone [n=18] and nitrofurantoin [n=17]; no case was attributed to octreotide).
- Levy-Khademi F, Irina S, Avnon-Ziv C, Levmore-Tamir M, Leder O. Octreotide-associated cholestasis and hepatitis in an infant with congenital hyperinsulinism. J Pediatr Endocrinol Metab 2015; 28: 449-51. [PubMed: 25324442](3 month of boy with congenital hyperinsulinemia had fluctuating liver test abnormalities on octreotide therapy [bilirubin rising to 20.1 mg/dL, direct 17 mg/dL, ALT 538 U/L, Alk P normal] and rapid recovery upon stopping).
- Visentin M, Stieger B, Merz M, Kullak-Ublick GA. Octreotide inhibits the bilirubin carriers organic anion transporting polypeptides 1B1 and 1B3 and the multidrug resistance-associated protein 2. J Pharmacol Exp Ther 2015; 355: 145-51. [PubMed: 26330539](Studies using cell lines and membrane vesicles showed that octreotide inhibited several membrane transport proteins which are involved in bilirubin uptake and secretion).
- Melmed S, Popovic V, Bidlingmaier M, Mercado M, van der Lely AJ, Biermasz N, Bolanowski M, et al. Safety and efficacy of oral octreotide in acromegaly: results of a multicenter phase III trial. J Clin Endocrinol Metab 2015; 100: 1699-708. [PubMed: 25664604](Among 155 patients with acromegaly switched from injectable therapy to oral octreotide, the majority had a durable beneficial effect and side effects were those typical of octreotide, 18 [12%] had a “hepatobiliary” disorder and 12 [8%] developed gallstones; while “clinically meaningful alterations were not observed in laboratory safety parameters”, although 2 patients are described who developed ALT elevations and jaundice).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was judged probably due to octreotide, a 67 year old woman with severe heart disease, diabetes and gastrointestinal bleeding developed marked cholestasis [peak bilirubin 49.3 mg/dL, ALT 533 U/L, Alk P 1482 U/L] while in the intensive care unit receiving octreotide and multiple other medications).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Lanreotide.[LiverTox: Clinical and Researc...]Review Lanreotide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Pasireotide.[LiverTox: Clinical and Researc...]Review Pasireotide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future.[Endocr Relat Cancer. 2016]Review Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future.Öberg K, Lamberts SW. Endocr Relat Cancer. 2016 Dec; 23(12):R551-R566. Epub 2016 Oct 3.
- Differing effects on gall-bladder motility of lanreotide SR and octreotide LAR for treatment of acromegaly.[Eur J Endocrinol. 1999]Differing effects on gall-bladder motility of lanreotide SR and octreotide LAR for treatment of acromegaly.Turner HE, Lindsell DR, Vadivale A, Thillainayagam AV, Wass JA. Eur J Endocrinol. 1999 Dec; 141(6):590-4.
- Assessment of gall bladder dynamics, cholecystokinin release and the development of gallstones during octreotide therapy for acromegaly.[Q J Med. 1992]Assessment of gall bladder dynamics, cholecystokinin release and the development of gallstones during octreotide therapy for acromegaly.Ewins DL, Javaid A, Coskeran PB, Shah S, Butler J, Deprez PH, Miell J, Calam J, Barrett JJ, Dawson JM. Q J Med. 1992 Apr; 83(300):295-306.
- Octreotide - LiverToxOctreotide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...