NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Odevixibat is an orally available inhibitor of the ilieal bile salt transporter which is used to treat severe pruritus in patients with cholestatic liver disease such as progressive familial intrahepatic cholestasis. Odevixibat is associated with transient serum enzyme elevations particularly with long term therapy but has not been linked to instances of clinically apparent liver injury with jaundice, although experience with its use has been limited.
Background
Odevixibat (oh” de vix' i bat) is a small molecule inhibitor of the ileal bile acid transporter and is used to treat the pruritus of severe cholestatic liver diseases such as progressive familial intrahepatic cholestasis (PFIC). Inhibition of the bile acid transporter blocks the reabsorption of bile salts in the terminal ileum and thereby lowers levels of serum bile acids that are raised in patients with PFIC. Three major forms of PFIC are known, due to genetic abnormalities in various hepatic biliary transporters: PFIC-1 due to the ATP8B1 protein (gene name FIC1), PFIC-2 to the bile acid exporter protein (BSEP: gene name ABCB11), and PFIC-3 to multi-drug resistant-3 protein (MDR3: gene name ABCB4). Children with PFIC develop progressive cholestatic liver injury with marked increases in serum total bile acids, variable degrees of symptoms of pruritus (itching) and fatigue which can be disabling. Most children have progressive cholestatic liver injury and eventually require liver transplantation. Odevixibat was approved for use in the United States in 2021 as therapy of severe pruritus in children with PFIC. It is under evaluation as therapy for other severe forms of cholestatic liver injury such as Alagille syndrome and biliary atresia, but initially was not formally approved for those uses. Odevixibat is available under the brand name Bylvay in capsules of 400 µg and 1200 µg to be swallowed as well as in 200 µg and 600 µg capsules to be opened and sprinkled on soft food as oral pellets. The recommended initial dose is 40 µg/kg once daily which can be increased to 100 µg/kg daily based upon tolerance and effect. Odevixibat therapy can be associated with symptoms of diarrhea, abdominal pain, nausea and vomiting, and serum aminotransferase elevations, but these adverse events are also common in untreated children with PFIC. Odevixibat acts locally on the bile acid transporter in the distal ileum and has little systemic absorption. Severe adverse events are rare, but long term therapy can be associated with fat-soluble vitamin deficiencies for which reason prospective monitoring of serum levels of these vitamins is recommended.
Hepatotoxicity
In trials of odevixibat in children with cholestatic liver diseases, serum ALT elevations of greater than 3 times ULN arose in 8% to 11% of treated participants and were particularly common with long term therapy. However, children with PFIC typically have serum ALT and AST elevations, and it was difficult to establish whether mild-to-moderate serum enzyme elevations were due to odevixibat therapy or to the spontaneous fluctuations that occur with the underlying disease. Clinically apparent liver injury with jaundice or hepatic decompensation has not been reported in children treated with odevixibat, although the total clinical experience with its use is limited.
Likelihood score: E* (suspected but unproven cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which odevixibat might cause liver injury is not known. It has little oral absorption and systemic levels are minimal. The serum aminotransferase elevations that occur with therapy may be due to its effects on absorption of fat soluble vitamins or medications, or to fluxes in the bile acid pool, or to changes in the intestinal microbiome.
Outcome and Management
While chronic therapy with odevixibat can be associated with mild-to-moderate serum aminotransferase elevations, it has not been linked to cases of clinically apparent liver injury. Because of the frequency of enzyme elevations detected during therapy, the product label for odevixibat recommends obtaining baseline liver tests before initiation of treatment and monitoring levels during therapy. Any ALT or AST elevation associated with symptoms or jaundice should lead to discontinuation. Moderate elevations above baseline without symptoms, however, can be managed with dose modification or interruption. There is no known cross sensitivity of the hepatic injury from odevixibat with other agents used to treat pruritus. Because of the similarity in chemical structure and mechanism of action, there may be cross sensitivity to side effects with maralixibat, another FDA approved ileal bile acid transporter inhibitor.
Drug Class: Ileal Bile Acid Transporter (IBAT) Inhibitors
Other Drugs in the Subclass, Pruritus Agents: Maralixibat
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Odevixibat – Bylvay®
DRUG CLASS
Ileal Bile Acid Transporter (IBAT) Inhibitors
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Odevixibat | 501692-44-0 | C37-H48-N4-O8-S2 |
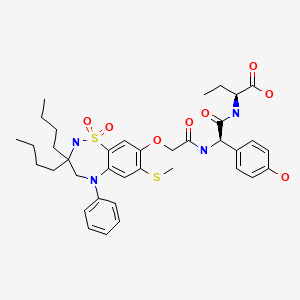
|
ANNOTATED BIBLIOGRAPHY
References updated: 10 March 2022
Abbreviations: PFIC, progressive familial intrahepatic cholestasis.
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 697-8.(Expert review of hepatotoxicity published in 1999 before the availability of inhibitors of the ileal bile acid transporter).
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Multi-authored textbook of hepatotoxicity published in 2013, does not discuss maralixibat or odevixibat).
- Fuchs CD, Halilbasic E, Trauner M. Pathophysiologic basis of alternative therapies for cholestasis. In, Arias IM, Alter HJ, Boyer JL, Cohen DE, Shafritz DA, Thorgeirsson SS, Wolkoff AW. The Liver: biology and pathobiology. 6th ed. Hoboken, NJ: Wiley Blackwell, 2020, pp 364-77.(Textbook of liver physiology and disease).
- FDA Medical Review of NDA for Odevixibat: Pages 79-86.
- https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2021/215498Orig1s000IntegratedR.pdf . (FDA website with product labels and integrated review of the efficacy and safety of odevixibat mentions that serum aminotransferase and bilirubin elevations were frequent in patients with PFIC at baseline, but that further increases were more frequent in odevixibat- than placebo-recipients, ALT in 9.5% vs 0% and bilirubin in 19% vs 5%, as well as treatment interruptions due to liver test abnormalities (16% vs 10%). - Kamath BM, Spino C, McLain R, Magee JC, Fredericks EM, Setchell KD, Miethke A. Met al.; Childhood Liver Disease Research Network (ChiLDReN). Unraveling the relationship between itching, scratch scales, and biomarkers in children with Alagille syndrome. Hepatol Commun. 2020;4:1012–1018. [PMC free article: PMC7327199] [PubMed: 32626833](Among 37 children with Alagille syndrome, instruments to measure pruritus [the symptom of itching vs the physical findings of scratching] correlated poorly with bile salt, bilirubin and GGT levels, but symptom of itching did correlate with some elements of quality of life including fatigue and sleep disturbance).
- Karpen SJ, Kelly D, Mack C, Stein P. Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders. Hepatol Int. 2020;14:677–689. [PubMed: 32653991](Review of the mechanism of action and rationale for use of ileal bile acid transporter inhibitors as therapy of cholestatic liver diseases in children, such as biliary atresia and Alagille syndrome).
- Slavetinsky C, Sturm E. Odevixibat and partial external biliary diversion showed equal improvement of cholestasis in a patient with progressive familial intrahepatic cholestasis. BMJ Case Rep. 2020;13:e234185. [PMC free article: PMC7326258] [PubMed: 32601135](15 month old boy with severe pruritus due to PFIC-2 [bile salt exporter protein deficiency) had marked decline in serum bile acids [124 to 6.5 µmol/L] and improvements in symptoms during a 4 week course of odevixibat, but return of laboratory abnormalities and symptoms with stopping, and then similar degree of improvements in bile acids [276 to <1 µmol/L] and symptoms after partial external biliary diversion).
- Baumann U, Sturm E, Lacaille F, Gonzalès E, Arnell H, Fischler B, Jørgensen MH, et al. Effects of odevixibat on pruritus and bile acids in children with cholestatic liver disease: Phase 2 study. Clin Res Hepatol Gastroenterol. 2021;45:101751. [PubMed: 34182185](Among 20 children [ages 1 to 17 years] with PFIC [n=13], Alagille syndrome [n=6], biliary atresia [n=3] or other severe cholestatic liver disease [n=2] treated with one of 5 doses of odevixibat [10, 30, 60, 100 or 200 µg/kg] daily for 4 weeks, bile acid levels decreased and symptoms improved in most patients while all patients completed therapy, there were no serious adverse events, and variable changes in ALT levels).
- Deeks ED. Odevixibat: first approval. Drugs. 2021;81:1781–1786. [PMC free article: PMC8550539] [PubMed: 34499340](Review of the history of development, mechanism of action, clinical efficacy, and safety of odevixibat shortly after its approval in the US for the pruritus of PFIC, mentions that a controlled trial of therapy in 62 subjects with PFIC reported adverse events in 33% on odevixibat vs 15% on placebo with ALT elevations in 8.7% to 10.5% on drug [40 and 100 µg/kg] vs 5% on placebo, but there were no episodes of hepatic decompensation or fat-soluble vitamin deficiency).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Effects of odevixibat on pruritus and bile acids in children with cholestatic liver disease: Phase 2 study.[Clin Res Hepatol Gastroenterol...]Effects of odevixibat on pruritus and bile acids in children with cholestatic liver disease: Phase 2 study.Baumann U, Sturm E, Lacaille F, Gonzalès E, Arnell H, Fischler B, Jørgensen MH, Thompson RJ, Mattsson JP, Ekelund M, et al. Clin Res Hepatol Gastroenterol. 2021 Sep; 45(5):101751. Epub 2021 Jun 26.
- Interim results from an ongoing, open-label, single-arm trial of odevixibat in progressive familial intrahepatic cholestasis.[JHEP Rep. 2023]Interim results from an ongoing, open-label, single-arm trial of odevixibat in progressive familial intrahepatic cholestasis.Thompson RJ, Artan R, Baumann U, Calvo PL, Czubkowski P, Dalgic B, D'Antiga L, Di Giorgio A, Durmaz Ö, Gonzalès E, et al. JHEP Rep. 2023 Aug; 5(8):100782. Epub 2023 Apr 29.
- Odevixibat and partial external biliary diversion showed equal improvement of cholestasis in a patient with progressive familial intrahepatic cholestasis.[BMJ Case Rep. 2020]Odevixibat and partial external biliary diversion showed equal improvement of cholestasis in a patient with progressive familial intrahepatic cholestasis.Slavetinsky C, Sturm E. BMJ Case Rep. 2020 Jun 29; 13(6). Epub 2020 Jun 29.
- Review Odevixibat: A Novel Bile Salt Inhibitor Treatment for Pruritus in Progressive Familial Intrahepatic Cholestasis.[Cureus. 2024]Review Odevixibat: A Novel Bile Salt Inhibitor Treatment for Pruritus in Progressive Familial Intrahepatic Cholestasis.Flattmann FE, Mohiuddin FS, Singh A, Tandon A, Lockett SJ, Hirsch JD, Mosieri CN, Kaye AM, Varrassi G, Ahmadzadeh S, et al. Cureus. 2024 Mar; 16(3):e56886. Epub 2024 Mar 25.
- Review Maralixibat.[LiverTox: Clinical and Researc...]Review Maralixibat.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Odevixibat - LiverToxOdevixibat - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...