NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Pasireotide is a synthetic polypeptide analogue of somatostatin that resembles the native hormone in its ability to suppress levels and activity of growth hormone, insulin, glucagon and many other gastrointestinal peptides. Because its half-life is longer than somatostatin, pasireotide can be used clinically to treat neuroendocrine pituitary tumors that secrete excessive amounts of growth hormone causing acromegaly, or adrenocorticotropic hormone (ACTH) causing Cushing disease. Pasireotide has many side effects including suppression of gall bladder contractility and bile production, and maintenance therapy can cause cholelithiasis and accompanying elevations in serum enzymes and bilirubin.
Background
Pasireotide (pa" ze ree' oh tide) is a synthetic octapeptide and analogue of somatostatin that is used for its ability to suppress levels and activities of hormones (growth hormone, insulin, gastrin, secretin, glucagon) or active neuropeptides (serotonin, vasoactive intestinal polypeptide [VIP]). Natural somatostatin is produced in the hypothalamus and acts to suppress growth hormone release from the pituitary. Somatostatin is also found in other neurons throughout the body and particularly in intestinal and pancreatic neurons, where it is active in suppressing release of hormones and neuropeptides such as insulin, glucagon, ACTH, gastrin, secretin, motilin, VIP, serotonin and cholecystokinin. Because of its short half-life (~3 minutes), somatostatin is impractical as a therapeutic agent, and analogues have been developed that have a more favorable pharmacological profile such as octreotide, pasireotide and lanreotide, all three of which have been marketed in long acting release (LAR) forms to allow once weekly or monthly administration. Pasireotide appears to interact largely with the somatostatin subtypes 1, 2 and 3 and possibly subtype 5 receptors, but otherwise acts in a similar manner to somatostatin. Pasireotide therapy has been shown to improve symptoms and complications of several neuroendocrine tumors including abnormal growth in acromegaly due to growth hormone secreting pituitary tumors and symptoms of excessive glucocorticoid production due to ACTH secreting pituitary tumors. Pasireotide was approved for use in treating Cushing disease in the United States in 2012 and a long acting form for as treatment of acromegaly in 2014. Pasireotide is available under the brand name Signifor in single dose ampules of 0.3, 0.6 and 0.8 mg in one mL solution for injection which is administered as a subcutaneous injection in doses of 0.3 to 0.9 mg twice daily (for Cushing disease). Pasireotide has been formulated also in a long acting release form under the brand name Signifor LAR, in 20, 40 and 60 mg vials of powder for reconstitution, the recommended dose (acromegaly) being 40 mg by intramuscular injection every 4 weeks with subsequent titration based upon efficacy and tolerance. Side effects are common. Adverse events from single injections include influenza-like symptoms of fatigue, headache, nausea and vomiting and local infusion reactions. With continued therapy, adverse events can include diarrhea, abdominal pain, back pain, headache, dizziness, hypothyroidism, hypo- and hyperglycemia, arrhythmias and gall bladder disease.
Hepatotoxicity
Mild, transient, asymptomatic elevations in serum aminotransferase levels occur in up to 29% of patients receiving pasireotide LAR, but elevations above 5 times the upper limit of normal are rare (<1%) and no case of clinically apparent liver injury with jaundice was reported in the preregistration trials of pasireotide in acromegaly. In some instances, the serum enzyme elevations appeared to reflect cholelithiasis and cholecystitis rather than hepatic injury and in all cases the abnormalities resolved, usually without dose modification or discontinuation. Similar or greater rates of liver test abnormalities were observed with octreotide LAR therapy. Since its approval and more widescale use, there have been no published reports of liver injury or hepatitis attributed to pasireotide. The product label for pasireotide mentions that hepatic serum enzyme elevations can occur on therapy and recommends monitoring “prior to and during therapy.”
Pasireotide causes inhibition of gall bladder contractility and a decrease in bile secretion, and long term therapy is associated with a high rate of cholesterol gallstone formation. In prospective studies, between 20% and 30% of patients with acromegaly treated with maintenance pasireotide for one to two years developed gallstones detected by ultrasonography and a proportion developed symptomatic cholelithiasis requiring hospitalization and cholecystectomy. Even after cholecystectomy, cholesterol stones may form in the common bile duct and intrahepatic ducts during somatostatin analogue therapy which can cause symptoms and liver test abnormalities. Therapy with ursodiol does not appear to prevent gallstone formation related to somatostatin analogue therapy, although it may help.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent hepatobiliary injury).
Mechanism of Injury
Pasireotide, like somatostatin, decreases cholecystokinin secretion, gall bladder contractility and bile secretion, perhaps accounting for the high rate of gall bladder sludge and stone formation with long term use. How infusions of pasireotide might cause acute liver injury independent of its effect on bile flow and gall bladder function is uncertain. Pasireotide is a polypeptide and, as such, should not have direct or even indirect hepatic toxicity. Thus, the liver injury, like the gall bladder effects, is likely caused by what pasireotide does, rather than by its chemical structure or what it is. Pasireotide has multiple effects on the gastrointestinal tract, including effects on gastrointestinal hormone levels, motility, transit time, bacterial flora, and bile acid concentrations.
Outcome and Management
The product label for pasireotide recommends evaluation of “liver enzyme test prior to and during treatment.” The liver test abnormalities during pasireotide therapy generally resolve rapidly with stopping therapy, but may resolve even with drug continuation. Patients should be monitored if pasireotide is restarted after liver test abnormalities resolve. Nevertheless, in many instances, pasireotide has been restarted without recurrence. There is little information on cross sensitivity to liver injury among the different somatostatin analogues, but liver injury has been reported most frequently with octreotide.
Drug Class: Hormonal Agents; Antineoplastic Agents; Gastrointestinal Agents
Other Drugs in the Subclass, Hormonal Agents, Somatostatin Analogues: Lanreotide, Octreotide
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pasireotide – Signifor®, Signifor LAR®
DRUG CLASS
Hormonal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lanreotide | 108736-35-2 | C54-H69-N11-O10-S2 |
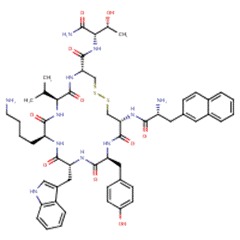 |
| Octreotide | 83150-76-9 | C49-H66-N10-O10-S2 |
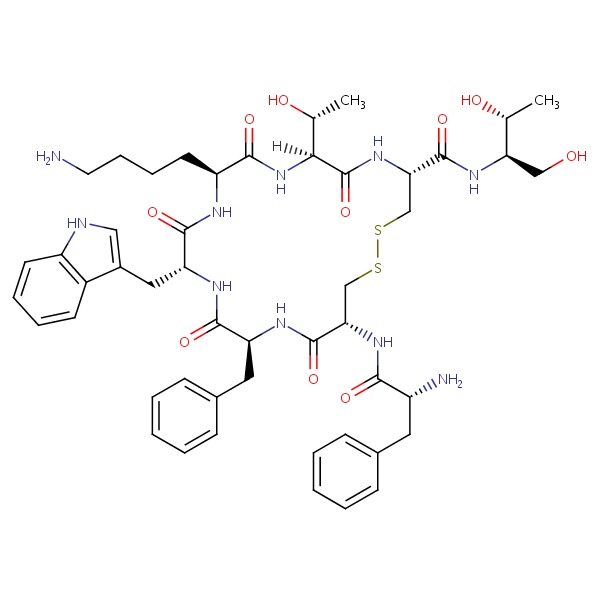 |
| Pasireotide | 396091-73-9 | C58-H66-N10-O9 |
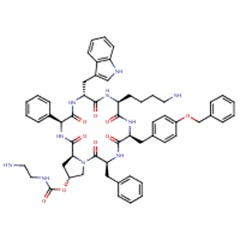 |
| Somatostatin | 51110-01-1 | Protein | Not Available |
ANNOTATED BIBLIOGRAPHY
References updated: 20 April 2017
- Zimmerman HJ. Hepatic injury from the treatment of infectious and parasitic diseases. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 589-637.(Expert review of hepatotoxicity published in 1999 and before the availability of pasireotide or lanreotide).
- Kaplowitz N, DeLeve LD, eds. Drug-induced Liver Disease. 3rd edition. Amsterdam: Elsevier, 2013.(Multiauthored text book on drug induced liver injury; does not discuss lanreotide or pasireotide).
- Sharkey KA, Wallace JL. Treatment of disorders of bowel motility and water flux: anti-emetics; agents used in biliary and pancreatic disease. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1323-49.(Textbook of pharmacology and therapeutics).
- Petersenn S, Schopohl J, Barkan A, Mohideen P, Colao A, Abs R, Buchelt A, et al.; Pasireotide Acromegaly Study Group. Pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, phase II trial. J Clin Endocrinol Metab 2010; 95: 2781-9. [PubMed: 20410233](Among 60 patients with acromegaly treated with 28 days of octreotide [three times daily] crossed over to pasireotide [200, 400 or 600 µcg twice daily], biochemical responses were more frequent with pasireotide and common adverse events were nausea [25%], diarrhea [22%], abdominal pain [12%] and diabetes [5%]; no mention of ALT elevations or serious liver related adverse events).
- Pasireotide (Signifor) for Cushing’s Disease. Med Lett Drugs Ther 2013; 55 (1416): 39-40. [PubMed: 23669783](Concise review of the mechanism of action, clinical efficacy, safety and costs of pasireotide shortly after its approval for use in Cushing disease in the US; mentions adverse reactions of diarrhea, nausea, cholelithiasis, abdominal pain, headache and fatigue and that hyperglycemia, hypercortisolism, QT prolongation, pituitary hormone deficiencies and hepatic enzyme elevations can also occur).
- Colao A, Bronstein MD, Freda P, Gu F, Shen CC, Gadelha M, Fleseriu M, et al.; Pasireotide C2305 Study Group. Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab 2014; 99: 791-9. [PMC free article: PMC3965714] [PubMed: 24423324](Among 358 patients with acromegaly and no previous medical therapy treated with monthly injections of either pasireotide or octreotide for 12 months, biochemical control of growth hormone and insulin-like growth factor levels were more frequent with pasireotide than octreotide as was hyperglycemia [29% vs 8%], while cholelithiasis arose commonly in both groups [26% vs 36%]; no mention of ALT elevations or hepatotoxicity).
- Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M, Pronin V, et al.; Pasireotide C2402 Study Group. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol 2014; 2: 875-84. [PubMed: 25260838](Among 198 patients with acromegaly inadequately controlled on octreotide or lanreotide crossed over to pasireotide or continued on previous regimen for 24 weeks, 15-20% of the pasireotide treated versus none continued on the previous regimen achieved biochemical control of the acromegaly, while adverse events included hyperglycemia in 31-33% of pasireotide vs 9% of active control subjects, cholelithiasis in 10% vs 12% and one subject on pasireotide required dose interruption because of abnormal liver test results [no details provided]).
- Sheppard M, Bronstein MD, Freda P, Serri O, De Marinis L, Naves L, Rozhinskaya L, et al. Pasireotide LAR maintains inhibition of GH and IGF-1 in patients with acromegaly for up to 25 months: results from the blinded extension phase of a randomized, double-blind, multicenter, Phase III study. Pituitary 2015; 18: 385-94. [PMC free article: PMC4424273] [PubMed: 25103549](Among patients with acromegaly who had a beneficial response to somatostatin analogue therapy in a prospective trial [Colao 1914] and were continued on therapy for 26 months, clinical responses were mostly maintained and no new adverse reactions were identified, 31% of pasireotide treated subjects developed hyperglycemia and 30% cholelithiasis; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to octreotide, but none to lanreotide or pasireotide).
- McKeage K. Pasireotide in acromegaly: a review. Drugs 2015; 75: 1039-48. [PubMed: 26017304](Review of the mechanism of action, pharmacology, clinical efficacy and safety of pasireotide as therapy of acromegaly; does not mention ALT elevations or hepatotoxicity).
- Bronstein MD, Fleseriu M, Neggers S, Colao A, Sheppard M, Gu F, Shen CC, et al.; Pasireotide C2305 Study Group. Switching patients with acromegaly from octreotide to pasireotide improves biochemical control: crossover extension to a randomized, double-blind, Phase III study. BMC Endocr Disord 2016; 16: 16. [PMC free article: PMC4818908] [PubMed: 27039081](Among the 358 patients with acromegaly enrolled in a controlled trial comparing pasireotide to octreotide for 12 months [Colao 2014], 119 did not achieve biochemical control and were crossed over to the other regimen for 12 months, and 17% of 81 switched to pasireotide vs none of 38 switched to octreotide had a biochemical response; while adverse event rates were similar, but hyperglycemia was more frequent with pasireotide [32% vs 13%] while cholelithiasis rates were similar [20% vs 19%]; no mention of ALT elevations or hepatoxicity).
- Öberg K, Lamberts SW. Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future. Endocr Relat Cancer 2016; 23: R551-R566. [PubMed: 27697899](Review of the use of somatostatin analogues such as oct-, pasi- and lan-reotide for acromegaly and neuroendocrine tumors; mentions that one-third to one-half of patients on somatostatin analogues can develop gallstones or biliary sludge).
- Fleseriu M, Rusch E, Geer EB; ACCESS Study Investigators. Safety and tolerability of pasireotide long-acting release in acromegaly-results from the acromegaly, open-label, multicenter, safety monitoring program for treating patients who have a need to receive medical therapy (ACCESS) study. Endocrine 2017; 55: 247-255. [PMC free article: PMC5225222] [PubMed: 27896545](Among 44 patients with acromegaly treated with pasireotide [40 mg every 28 days] for 4-70 weeks, hyperglycemia requiring antidiabetic therapy developed in 48% and there were 8 episodes of cholelithiasis, but there were apparently no liver related adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Lanreotide.[LiverTox: Clinical and Researc...]Review Lanreotide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Octreotide.[LiverTox: Clinical and Researc...]Review Octreotide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Pasireotide--a somatostatin analog for the potential treatment of acromegaly, neuroendocrine tumors and Cushing's disease.[IDrugs. 2007]Pasireotide--a somatostatin analog for the potential treatment of acromegaly, neuroendocrine tumors and Cushing's disease.Ben-Shlomo A, Melmed S. IDrugs. 2007 Dec; 10(12):885-95.
- Review Pasireotide - Mechanism of Action and Clinical Applications.[Curr Drug Metab. 2018]Review Pasireotide - Mechanism of Action and Clinical Applications.Sawicka-Gutaj N, Owecki M, Ruchala M. Curr Drug Metab. 2018; 19(10):876-882.
- Review Pasireotide, a multi-somatostatin receptor ligand with potential efficacy for treatment of pituitary and neuroendocrine tumors.[Drugs Today (Barc). 2013]Review Pasireotide, a multi-somatostatin receptor ligand with potential efficacy for treatment of pituitary and neuroendocrine tumors.Feelders RA, de Herder WW, Neggers SJ, van der Lely AJ, Hofland LJ. Drugs Today (Barc). 2013 Feb; 49(2):89-103.
- Pasireotide - LiverToxPasireotide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...