NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lanreotide is a synthetic polypeptide analogue of somatostatin that resembles the native hormone in its ability to suppress levels and activity of growth hormone, insulin, glucagon and many other gastrointestinal peptides. Because its half-life is longer than somatostatin, lanreotide can be used clinically to treat neuroendocrine tumors that secrete excessive amounts of growth hormone (acromegaly) or other active hormones or neuropeptides. Lanreotide has many side effects including suppression of gall bladder contractility and bile production, and maintenance therapy may cause cholelithiasis and pancreatitis as well accompanying liver injury.
Background
Lanreotide (lan ree' oh tide) is a synthetic octapeptide and analogue of somatostatin that is used for its ability to suppress levels and activities of hormones (growth hormone, insulin, gastrin, secretin, glucagon) or active neuropeptides (serotonin, vasoactive intestinal polypeptide [VIP]). Natural somatostatin is produced in the hypothalamus and acts to suppress growth hormone release from the pituitary. Somatostatin is also found in other neurons throughout the body and particularly in intestinal and pancreatic neurons, where it is active in suppressing release of hormones and neuropeptides such as insulin, glucagon, gastrin, secretin, motilin, VIP, serotonin and cholecystokinin. Because of its short half-life (~3 minutes), somatostatin is impractical as a therapeutic agent, and analogues have been developed that have a more favorable pharmacological profile such as octreotide, pasireotide and lanreotide, all three of which have been marketed in long acting release (LRA) forms to allow once weekly or monthly administration. Lanreotide appears to interact largely with the somatostatin subtype 2 and possibly subtype 5 receptors, with little effect on subtypes 1, 3 and 4, but otherwise acts in a similar manner to somatostatin. Lanreotide therapy has been shown to improve symptoms and complications of several neuroendocrine tumors including abnormal growth in acromegaly due to growth hormone-secreting pituitary tumors, diarrhea due to VIP-secreting intestinal tumors, and flushing due to serotonin-producing carcinoid tumors. Lanreotide was approved for use in the United States in 2007 and current listed indications include acromegaly and unresectable, locally advanced or metastatic gastroenteropancreatic neuroendocrine tumors. Lanreotide has been used off label for polycystic liver and kidney disease. A long acting form of lanreotide is available under the brand name Somatuline Depot in prefilled syringes of 60 mg in 0.2 mL, 90 mg in 0.3 mL or 120 mcg in 0.5 mL (240 mg/mL). Lanreotide depot is administered as a deep subcutaneous injection every 4 weeks in doses of 60 to 120 mg each. Side effects are common. Adverse events from single injections include influenza-like symptoms of fatigue, headache, nausea and vomiting and local injection reactions. With continued therapy, common adverse events include diarrhea, abdominal pain, back pain, headache, dizziness, hypothyroidism, hypo- and hyperglycemia, arrhythmias and gallbladder disease.
Hepatotoxicity
In preregistration studies of lanreotide, serum enzyme levels did not change appreciably and there were no reports of clinically apparent acute liver injury. Pooled analyses reported that there were no overall changes in serum ALT, AST or alkaline phosphatase levels during therapy or instances of clinically meaningful elevations with treatment. Prolonged therapy with lanreotide, as with other somatostatin analogues, was associated with a high rate of biliary sludge and cholelithiasis, probably due to inhibition of gall bladder contractility and decrease in bile secretion. In long term studies, cholelithiasis developed in 20% to 33% of lanreotide treated patients. In some instances, symptomatic cholecystitis occurred which can be accompanied by mild-to-moderate elevations in serum enzymes and bilirubin. However, most lanreotide associated gallstones were asymptomatic. Unlike octreotide, lanreotide and other long acting somatostatin analogues have not been liked to cases of clinically apparent liver injury, independent of cholelithiasis or biliary sludge, although they have had more limited use and have not been used in many of the clinical situations that were treated with octreotide (portal hypertension, variceal hemorrhage and infants with congenital hyperinsulinemia).
Likelihood score: E* (unproven but suspected rare cause of clinically apparent hepatobiliary injury).
Mechanism of Injury
Lanreotide, like somatostatin, decreases cholecystokinin secretion, gall bladder contractility and bile secretion, perhaps accounting for the high rate of gall bladder sludge and stone formation with long term use. How lanreotide might cause acute liver injury independent of its effect on bile flow and gall bladder function is uncertain. Lanreotide is a polypeptide and, as such, should not have direct or even indirect hepatic toxicity. On the other hand, lanreotide has multiple effects on the gastrointestinal tract, including effects on gastrointestinal hormone levels, motility, transit time, bacterial flora, and bile acid concentrations, all of which may have indirect effects on the liver.
Outcome and Management
The liver injury due to the lanreotide is generally due to its effects on the gallbladder and bile flow. Patients with hepatobiliary complications of lanreotide therapy are likely to have recurrence with restarting and have similar response to other somatostatin analogues such as octreotide and pasireotide.
Drug Class: Hormonal Agents; Antineoplastic Agents; Gastrointestinal Agents
Other Drugs in the Subclass, Hormonal Agents, Somatostatin Analogues: Octreotide, Pasireotide
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Lanreotide – Somatuline Depot®
DRUG CLASS
Hormonal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lanreotide | 108736-35-2 | C54-H69-N11-O10-S2 |
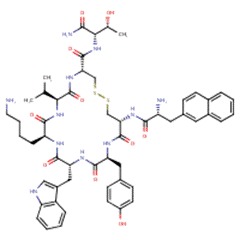 |
| Octreotide | 83150-76-9 | C49-H66-N10-O10-S2 |
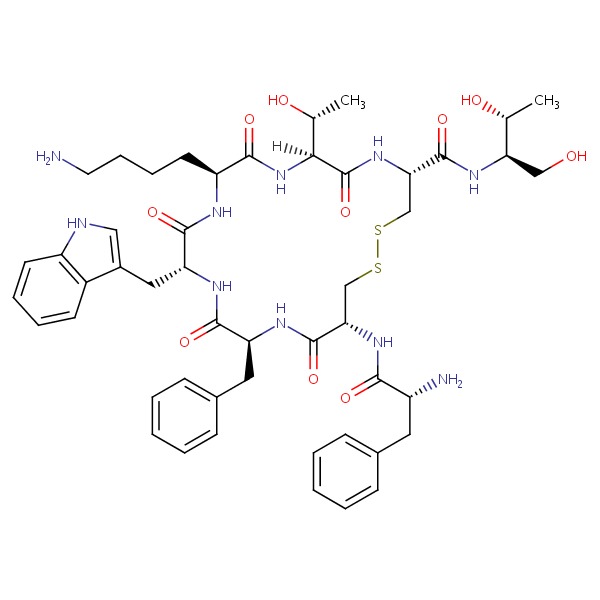 |
| Pasireotide | 396091-73-9 | C58-H66-N10-O9 |
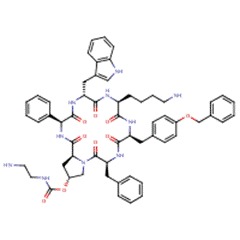 |
| Somatostatin | 51110-01-1 | Protein | Not Available |
ANNOTATED BIBLIOGRAPHY
References updated: 11 April 2017
- Zimmerman HJ. Hepatic injury from the treatment of infectious and parasitic diseases. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 589-637.(Expert review of hepatotoxicity published in 1999 and before the availability of lanreotide).
- Kaplowitz N, DeLeve LD, eds. Drug-induced Liver Disease. 3rd edition. Amsterdam: Elsevier, 2013.(Multiauthored text book on drug induced liver injury; does not discuss lanreotide).
- Sharkey KA, MacNaughton WK. Gastrointestinal motility and water flux, emesis and biliary and pancreatic disease. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 921-44.(Textbook of pharmacology and therapeutics).
- Caron P, Morange-Ramos I, Cogne M, Jaquet P. Three-year follow-up of acromegalic patients treated with intramuscular slow-release lanreotide. J Clin Endocrinol Metab 1997; 82: 18-22. [PubMed: 8989225](Among 22 acromegalic patients treated with lanreotide every 10-14 days for at least 3 years, 13 had minor digestive symptoms during the 48 hours after each injection and 4 developed gallstones).
- van Keimpema L, Nevens F, Vanslembrouck R, van Oijen MG, Hoffmann AL, Dekker HM, de Man RA, Drenth JP. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2009; 137: 1661-8. [PubMed: 19646443](Among 54 patients with polycystic liver disease treated with lanreotide or placebo every 4 weeks for 24 weeks, the average liver size decreased by 2.9% with lanreotide and increased with placebo, while side effects were limited to 1-4 days of loose stools after each injection and there were no changes in routine liver test results).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were due to a somatostatin analogue).
- Chrispijn M, Nevens F, Gevers TJ, Vanslembrouck R, van Oijen MG, Coudyzer W, Hoffmann AL, et al. The long-term outcome of patients with polycystic liver disease treated with lanreotide. Aliment Pharmacol Ther 2012; 35: 266-74. [PubMed: 22111942](Among 41 patients who completed a placebo controlled study of 6 months of lanreotide for polycystic liver disease [van Keimpema 2009] and who were continued on drug [120 mg per month] for another 12 months, liver size did not decrease further while “liver enzyme values remained constant”).
- Temmerman F, Gevers T, Ho TA, Vanslembrouck R, Coudyzer W, van Pelt J, Bammens B, et al. Safety and efficacy of different lanreotide doses in the treatment of polycystic liver disease: pooled analysis of individual patient data. Aliment Pharmacol Ther 2013; 38: 397-406. [PubMed: 23799922](In a pooled analysis of studies of lanreotide therapy of polycystic liver disease, higher doses [120 vs 90 mg] were associated with greater reduction in liver size, but adverse event rates were similar; no mention of ALT elevations or cholelithiasis).
- Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, et al.; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014; 371: 224-33. [PubMed: 25014687](Among 204 patients with metastatic neuroendocrine tumors treated with lanreotide or placebo every 4 weeks for 96 weeks, progression free survival was prolonged by lanreotide, but the overall rates of adverse events were similar; those more frequent with lanreotide were diarrhea, abdominal pain, cholelithiasis(10%], flatulence, nausea, headache and lethargy; one patient developed “liver decompensation” the day after the first injection, but ultimately recovered).
- Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Wetzels JF, Drenth JP. Effect of lanreotide on polycystic liver and kidneys in autosomal dominant polycystic kidney disease: an observational trial. Liver Int 2015; 35: 1607-14. [PubMed: 25369108](Among 43 patients with autosomal dominant polycystic kidney disease treated with lanreotide for at least 6 months, mean liver volume decreased by an average of 3.1% and there were no “clinically relevant or statistically significant changes” in laboratory results and no patient developed symptomatic cholecystitis).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to octreotide, but none to lanreotide).
- Sagvand BT, Khairi S, Haghshenas A, Swearingen B, Tritos NA, Miller KK, Klibanski A, et al. Monotherapy with lanreotide depot for acromegaly: long-term clinical experience in a pituitary center. Pituitary 2016; 19: 437-47. [PubMed: 27155600](Compared to 39 surgically cured patients with acromegaly, 24 who were treated with lanreotide depot for at least 24 months were more likely to have gastrointestinal symptoms such as diarrhea, abdominal pain, and nausea and 9% [vs 0%] developed gallstones, but there were no significant changes in ALT, AST or alkaline phosphatase levels and no episodes of clinically apparent liver injury).
- Öberg K, Lamberts SW. Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future. Endocr Relat Cancer 2016; 23: R551-R566. [PubMed: 27697899](Review of the use of somatostatin analogues such as oct-, pasi- and lan-reotide for acromegaly and neuroendocrine tumors; mentions that one-third to one-half of patients on somatostatin analogues can develop gallstones or biliary sludge).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Octreotide.[LiverTox: Clinical and Researc...]Review Octreotide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Pasireotide.[LiverTox: Clinical and Researc...]Review Pasireotide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Differing effects on gall-bladder motility of lanreotide SR and octreotide LAR for treatment of acromegaly.[Eur J Endocrinol. 1999]Differing effects on gall-bladder motility of lanreotide SR and octreotide LAR for treatment of acromegaly.Turner HE, Lindsell DR, Vadivale A, Thillainayagam AV, Wass JA. Eur J Endocrinol. 1999 Dec; 141(6):590-4.
- Effectiveness and tolerability of slow release lanreotide treatment in active acromegaly: six-month report on an Italian multicenter study. Italian Multicenter Slow Release Lanreotide Study Group.[J Clin Endocrinol Metab. 1996]Effectiveness and tolerability of slow release lanreotide treatment in active acromegaly: six-month report on an Italian multicenter study. Italian Multicenter Slow Release Lanreotide Study Group.Giusti M, Gussoni G, Cuttica CM, Giordano G. J Clin Endocrinol Metab. 1996 Jun; 81(6):2089-97.
- Review Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future.[Endocr Relat Cancer. 2016]Review Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future.Öberg K, Lamberts SW. Endocr Relat Cancer. 2016 Dec; 23(12):R551-R566. Epub 2016 Oct 3.
- Lanreotide - LiverToxLanreotide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...