NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Fludarabine is a purine analogue and antineoplastic agent used in the therapy of chronic lymphocytic leukemia (CLL) and in immunosuppressive regimens in preparation of hematopoietic cell transplantation (HCT). Fludarabine is associated with a low rate of transient serum enzyme elevations during therapy and has only rarely been implicated in cases of clinically apparent acute liver injury with jaundice. Fludarabine has potent immunosuppressive activity and has been associated with many cases of reactivation of hepatitis B.
Background
Fludarabine (floo dar' a been) phosphate is a purine analogue that is used commonly in the treatment of chronic lymphocyte leukemia. Fludarabine phosphate is a fluorinated derivative of the antiviral agent adenine arabinoside monophosphate (2-fluoro-ara-AMP). Fludarabine is converted intracellularly to the active triphosphate, which competes with adenine triphosphate for use by DNA polymerase leading to inhibition of DNA synthesis. Fludarabine was found to have activity against several forms of leukemia and lymphoma and was approved for use as an antineoplastic agent in the United States in 1991. Current indications are therapy of chronic lymphocyte leukemia. Fludarabine is also used off-label for hairy cell leukemia, mycosis fungoides, and both Hodgkin and non-Hodgkin lymphomas as well as in immunosuppressive regimens for nonmyeloablative hematopoietic cell transplantation (HCT). Fludarabine is available as a powder or solution for injection generically and under the trade name Fludara. Oral formulations are also available as tablets of 10 mg generically and under the trade name Oforta. The typical adult dose for CLL is 25 mg/m2 intravenously or 40 mg/m2 orally each day for 5 days; each 5 day course given is every 28 days. The dose regimen for nonmyeloablative HCT is typically 30 mg/m2 intravenously for 3 days in the week before transplant, usually in combination with total body irradiation or other alkylating agents. Common side effects include bone marrow suppression, leucopenia, infections, fever, nausea, vomiting, anorexia, diarrhea, headache, fatigue and skin rash.
Hepatotoxicity
In clinical trials, serum enzymes elevations occurred in only a small proportion of patients treated with fludarabine for leukemia. The role of fludarabine as opposed to other antineoplastics used in antileukemic regimens was not always clear from these studies. Cases of clinically apparent liver injury due to fludarabine have been reported to occur, but few details were available and most patients were receiving other cancer chemotherapeutic agents.
Fludarabine is immunosuppressive and decreases total white blood cell counts and specifically lymphocyte counts and CD8 T cells. As a consequence, fludarabine therapy has been linked to cases of reactivation of chronic hepatitis B, including instances of reverse seroconversion marked by development of HBsAg and active disease in a patient who had resolved hepatitis B before chemotherapy, as shown by presence of anti-HBc without HBsAg. Reactivation typically occurs after 3 to 6 courses of anticancer mediations and most commonly 2 to 4 months after completing chemotherapy. The frequency and severity of reactivation after fludarabine therapy has led to recommendations that patients be screened for HBsAg and anti-HBc before treatment, and give prophylaxis with antiviral therapy using an oral nucleoside with potent activity against HBV, such as lamivudine, tenofovir or entecavir. If prophylaxis is not used, careful monitoring and early institution of antiviral therapy is warranted. Fludarabine has also been associated with development of opportunistic infections including herpes virus and adenovirus infection of the liver.
Likelihood score: E* (Unproven but suspected cause of clinically apparent liver injury).
Mechanism of Injury
Hepatotoxicity from fludarabine is likely due to direct toxicity. Reactivation of hepatitis B is likely due to the immunosuppressive activity of fludarabine. The specific role of fludarabine in situations in which patients also receive corticosteroids, rituximab or HCT cannot always be determined.
Outcome and Management
The severity of the liver injury linked to fludarabine therapy is generally self-limited and mild, and resolves with stopping therapy. Reactivation of hepatitis B, in contrast, can be severe and lead to acute liver failure and death.
Drug Class: Antineoplastic Agents, Antimetabolites
Other Drugs in the Subclass, Purine Analogues: Azathioprine, Cladribine, Clofarabine, Mercaptopurine, Nelarabine, Pentostatin, Thioguanine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Fludarabine (Injectable) – Generic, Fludara®
Fludarabine (Oral) – Generic, Oforta®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
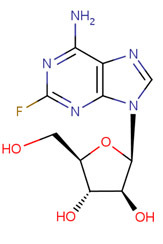
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Fludarabine | 21679-14-1 | C10-H12-F-N5-O4 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 01 February 2018
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999 mentions that fludarabine and cladribine were newly developed antineoplastic purine analogues and had yet to be implicated in causing liver injury).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 541-68.(Review of hepatotoxicity of hepatotoxicity of anticancer agents does not discuss fludarabine).
- Chabner BA, Bertino J, Cleary J, Ortiz T, Lane A, Supko JG, Ryan DP. Purine analogs. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1701-5.(Textbook of pharmacology and therapeutics).
- Harvey WH, Fleming TR, Beltran G, Saiers JH, Oishi N, Von Hoff DD. Phase II study of fludarabine phosphate in previously untreated patients with hepatoma: a Southwest Oncology Group Study. Cancer Treat Rep 1987; 71: 1111-2. [PubMed: 2445482](Among 19 patients with hepatocellular carcinoma treated with fludarabine [25 mg/m2/day for 5 days every 28 days], none had a clinical response and side effects were limited to myelosuppression, somnolence, peripheral neuropathy, nausea, vomiting, diarrhea and rash; no mention of ALT elevations or hepatotoxicity).
- Harvey WH, Fleming TR, Von Hoff DD, Katterhagen JG, Coltman CA Jr. Phase II study of fludarabine phosphate in previously untreated patients with colorectal carcinoma: a Southwest Oncology Group Study. Cancer Treat Rep. 1987; 71: 1319-20. [PubMed: 2446754](Among 20 patients with advanced colorectal carcinoma treated with fludarabine [25 mg/m2/day for 5 days every 28 days], none had a clinical response and side effects were limited to myelosuppression, somnolence, nausea, vomiting, diarrhea and rash; no mention of ALT elevations or hepatotoxicity).
- Keating MJ, Kantarjian H, Talpaz M, Redman J, McCredie KB. Fludarabine therapy in chronic lymphocytic leukemia (CLL). Nouv Rev Fr Hematol 1988; 30 (5-6): 461-6. [PubMed: 2464794](Among 75 previously treated patients with CLL who were treated with fludarabine, 56% had a partial or complete response and the major toxicities were infections, leucopenia, neuropathy, nausea, stomatitis and diarrhea; no mention of ALT elevations or hepatotoxicity).
- Keating MJ, Kantarjian H, Talpaz M, Redman J, Koller C, Barlogie B, Velasquez W, et al. Fludarabine: a new agent with major activity against chronic lymphocytic leukemia. Blood 1989; 74: 19-25. [PubMed: 2473795](In preliminary studies of fludarabine in 68 previously treated patients with CLL, 56% had a partial or complete response and the major toxicities were myelosuppression and infections; one patient stopped therapy early because of "hepatitis").
- Chun HG, Leyland-Jones B, Cheson BD. Fludarabine phosphate: a synthetic purine antimetabolite with significant activity against lymphoid malignancies. J Clin Oncol 1991; 9: 175-88. [PubMed: 1702143](Review of results of treating more than 1500 patients with various malignancies with fludarabine, found promising results in chronic lymphocytic leukemia but little activity in common solid tumors; toxicities included myelosuppression and occasional elevations in serum aminotransferase levels).
- O'Brien S, Kantarjian H, Beran M, Koller C, Talpaz M, Lerner S, Keating MJ. Fludarabine and granulocyte colony-stimulating factor (G-CSF) in patients with chronic lymphocytic leukemia. Leukemia 1997; 11: 1631-5. [PubMed: 9324281](Among 25 adults with CLL treated with fludarabine [30 mg/m2 daily for 5 days, 1-9 courses] and G-CSF [starting on day 6], rates of neutropenia were less compared to historical controls, but rates of response and infection, except for pneumonia, were not different; no mention of ALT elevations or liver injury).
- Hogan WJ, Edwards WD, Macon WR, Habermann TM. Fulminant hepatic failure secondary to adenovirus following fludarabine-based chemotherapy for non-Hodgkin's lymphoma. Leuk Lymphoma 2001; 42: 1145-50. [PubMed: 11697635](54 year old man with non-Hodgkin lymphoma developed fever followed by respiratory and hepatic failure shortly after a third cycle of fludarabine and cyclophosphamide [bilirubin 6.5 mg/dL, AST 8838 U/L, Alk P 304 U/L], autopsy showing massive hepatic necrosis and presence of large intranuclear viral inclusions shown to harbor adenovirus by in situ hybridization).
- Ng HJ, Lim LC. Fulminant hepatitis B virus reactivation with concomitant listeriosis after fludarabine and rituximab therapy: case report. Ann Hematol 2001; 80 (9): 549-52. [PubMed: 11669307](53 year old woman with CLL and HBsAg carrier state developed jaundice and acute HBV reactivation 6 months after finishing 6 courses of fludarabine and cyclophosphamide, and 2 months after receiving 4 doses of rituximab [bilirubin 10.8 mg/dL, ALT 1479 U/L, Alk P 147 U/L, HBV DNA positive], with progressive liver failure, sepsis and death 12 days after presentation).
- Picardi M, Pane F, Quintarelli C, De Renzo A, Del Giudice A, De Divitiis B, Persico M, et al. Hepatitis B virus reactivation after fludarabine-based regimens for indolent non-Hodgkin's lymphomas: high prevalence of acquired viral genomic mutations. Haematologica 2003; 88: 1296-303. [PubMed: 14607759](Among 40 patients with non-Hodgkin lymphoma treated with fludarabine, reactivation of hepatitis B occurred in all 4 who were HBsAg positive before therapy, but in only 1 of 8 who had anti-HBc, anti-HBs or anti-HBe without HBsAg, arising after 4-16 weeks after completing therapy [peak bilirubin 6-22 mg/dL, ALT 800-2000 U/L], 2 dying and 3 recovering, all three becoming HBsAg negative).
- Hogan WJ, Maris M, Storer B, Sandmaier BM, Maloney DG, Schoch HG, Woolfrey AE, et al. Hepatic injury after nonmyeloablative conditioning followed by allogeneic hematopoietic cell transplantation: a study of 193 patients. Blood 2004; 103: 78-84. [PubMed: 12969980](Among 193 patients treated with nonmyeloablative HCT for malignancy 74% of whom received fludarabine [30 mg/m2 for 3 days], 51 [26%] developed jaundice [bilirubin >4 mg/dL] which was attributed to graft-vs-host disease [41%], sepsis, cyclosporine toxicity, hemolysis, ischemia, or metastases).
- Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, Sandmaier BM, et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood 2004; 104: 1550-8. [PubMed: 15150081](Comparison of 73 patients undergoing nonmyeloablative to 73 undergoing ablative preparation for HCT found a lower rate of severe hepatic toxicity after nonablative [16%, no sinusoidal obstruction syndrome:SOS] than ablative [31%; 18% SOS] regimens).
- Huynh T, Mansberg R, Hsiao E, Coyle L, Roach P. Transient absence of hepatic uptake on gallium-67 scintigraphy following fludarabine therapy. Clin Nucl Med 2004; 29: 275-6. [PubMed: 15096981](61 year old man with CLL developed fever and abnormal liver tests [no details provided] 2 weeks after finishing fludarabine therapy and had transient lack of hepatic update of gallium by scintigraphy, restored after 5 days).
- Kusumi E, Kami M, Kanda Y, Murashige N, Seki K, Fujiwara M, Koyama R, et al. Hepatic injury following reduced intensity unrelated cord blood transplantation for adult patients with hematological diseases. Biol Blood Marrow Transplant 2006; 12: 1302-9. [PubMed: 17162212](Among 104 adults undergoing reduced intensity preparation for HCT, 87% developed hyperbilirubinemia, but the abnormalities were not attributed to fludarabine, but rather graft-vs-host disease, cholangitis lenta, sepsis, concurrent drug therapy, disease progression and liver congestion).
- Wasmuth JC, Fischer HP, Sauerbruch T, Dumoulin FL. Fatal acute liver failure due to reactivation of hepatitis B following treatment with fludarabine/cyclophosphamide/rituximab for low grade non-Hodgkin's lymphoma. Eur J Med Res 2008; 13: 483-6. [PubMed: 19008178](55 year old man with non-Hodgkin lymphoma and "past history of hepatitis B" developed jaundice two months after completing 6 cycles of rituximab, fludarabine and cyclophosphamide [bilirubin 5.9 rising to 29.7 mg/dL, ALT 271 rising to 1374 U/L, Alk P 73 rising to 214 U/L, HBsAg, HBeAg and HBV DNA positive], dying of hepatic failure 1 month after presentation).
- Sanchez MJ, Buti M, Homs M, Palacios A, Rodriguez-Frias F, Esteban R. Successful use of entecavir for a severe case of reactivation of hepatitis B virus following polychemotherapy containing rituximab. J Hepatol 2009; 51: 1091-6. [PubMed: 19836097](62 year old man with CLL developed reverse seroconversion and reactivation of hepatitis B after 4 rounds of chemotherapy with fludarabine, cyclosphosphamide and rituximab [bilirubin 10.3, ALT 3481 U/L, INR 1.8, HBsAg, and HBV DNA positive], treated with entecavir and ultimately resolving the hepatitis B with loss of HBsAg).
- Power JP, El Chaar M, Temple J, Thomas M, Spillane D, Candotti D, Allain JP. HBV reactivation after fludarabine chemotherapy identified on investigation of suspected transfusion-transmitted Hepatitis B virus. J Hepatol 2010; 53: 780-7. [PubMed: 20638744](54 year old man with CLL and previous hepatitis B was treated with fludarabine and developed reverse seroconversion [bilirubin 1.7 mg/dL, ALT 104 U/L, HBsAg and HBeAg positive], resolving in follow up with clearance of HBsAg and development of anti-HBs).
- Pai RK, van Besien K, Hart J, Artz AS, O'Donnell PH. Clinicopathologic features of late-onset veno-occlusive disease/sinusoidal obstruction syndrome after high dose intravenous busulfan and hematopoietic cell transplant. Leuk Lymphoma 2012; 53: 1552-7. [PMC free article: PMC4482341] [PubMed: 22280517](Clinical description of 8 patients with delayed SOS after HCT using conditioning with busulfan, fludarabine and alemtuzumab with onset 37-77 days after transplant [peak bilirubin 1.3-22.9 mg/dL], most dying of persistent disease or SOS).
- Freedberg DE, Bhadelia N, Poneros JM, Oster MW. Visceral varicella in a patient with chronic lymphocytic leukemia treated with fludarabine: a case report. Clin Lymphoma Myeloma Leuk 2013; 13: 90-2. [PubMed: 23103084](54 year old man with CLL on fludarabine therapy developed varicella-zoster with involvement of intestine, pancreas and liver including ALT elevations [twice ULN], resolving with valacyclovir therapy).
- Ronan BA, Agrwal N, Carey EJ, De Petris G, Kusne S, Seville MT, Blair JE, Vikram HR. Fulminant hepatitis due to human adenovirus. Infection 2014; 42: 105-11. [PubMed: 23979854](70 year old woman with CLL treated with fludarabine, rituximab and prednisone developed fever and rising liver tests [bilirubin rising to 5.4 mg/dL, ALT 1699 U/L, Alk P 173 U/L], progressing to multiorgan failure and death; autopsy showed adenovirus hepatitis by in situ hybridization).
- Chen S, Osborn JD, Chen X, Boyer MW, McDonald GB, Hildebrandt GC. Subacute hepatic necrosis mimicking veno-occlusive disease in a patient with HFE H63D homozygosity after allogeneic hematopoietic cell transplantation with busulfan conditioning. Int J Hematol 2015; 102: 729-31. [PubMed: 26497867](31 year old man with myelodysplasia underwent hematopoietic cell transplanation after myeloablation using busulfan and fludarabine and developed fever and liver injury 23 days after transplant [bilirubin 1.3 mg/dL, ALT 1339 U/L, Alk P 84 U/L, INR 1.3], and SOS on liver biopsy, resolving within the following 2 weeks).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, anticancer agents accounted for 49 cases [6%] but none were attributed to fludarabine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Purine Analogues.[LiverTox: Clinical and Researc...]Review Purine Analogues.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Venetoclax.[LiverTox: Clinical and Researc...]Review Venetoclax.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Pentostatin (Nipent) and chlorambucil with granulocyte-macrophage colony-stimulating factor support for patients with previously untreated, treated, and fludarabine-refractory B-cell chronic lymphocytic leukemia.[Semin Oncol. 2000]Review Pentostatin (Nipent) and chlorambucil with granulocyte-macrophage colony-stimulating factor support for patients with previously untreated, treated, and fludarabine-refractory B-cell chronic lymphocytic leukemia.Waselenko JK, Grever MR, Beer M, Lucas MA, Byrd JC. Semin Oncol. 2000 Apr; 27(2 Suppl 5):44-51.
- Review Treatment of fludarabine-refractory chronic lymphocytic leukemia.[Cancer. 2009]Review Treatment of fludarabine-refractory chronic lymphocytic leukemia.Tsimberidou AM, Keating MJ. Cancer. 2009 Jul 1; 115(13):2824-36.
- Review Purine analogs in chronic lymphocytic leukemia and Waldenström's macroglobulinemia.[Ann Oncol. 1996]Review Purine analogs in chronic lymphocytic leukemia and Waldenström's macroglobulinemia.O'Brien S, Kantarjian H, Keating MJ. Ann Oncol. 1996; 7 Suppl 6:S27-33.
- Fludarabine - LiverToxFludarabine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...