NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Flucytosine is an antifungal agent used to treat severe infections caused by candida and cryptococcus. Flucytosine therapy can cause transient mild-to-moderate serum aminotransferase elevations and has been mentioned as a very rare cause of clinically apparent acute drug induced liver injury.
Background
Flucytosine (floo sye' toe zeen) is a fluorinated pyrimidine analogue which has potent fungicidal activity. Flucytosine is taken up by fungal cells and converted to fluorouracil, which blocks pyrimidine metabolism and may be converted to metabolites that block DNA synthesis. Human cells do not possess the enzymes to convert flucytosine to these toxic metabolites. Flucytosine is active against several Candidal and Cryptococcal species. Flucytosine was approved for use in the United States in 1971, but has been largely replaced by better tolerated and more potent antifungal agents such as amphotericin and the azoles. Flucytosine is available as tablets of 250 and 500 mg in generic forms and under the brand name of Ancobon. The usual recommended dose is 50 to 150 mg/kg daily in divided doses (usually every six hours) and it is usually used in combination with other antifungal agents, most often amphotericin B. Common side effects include nausea, vomiting, and headache. Rare but severe side effects include bone marrow suppression, renal failure, cardiac arrest and toxic epidermal necrolysis.
Hepatotoxicity
Transient mild-to-moderate elevations in serum aminotransferase or alkaline phosphatase levels occur in up to 41% of patients treated with flucytosine. The enzyme abnormalities are usually asymptomatic and resolve with stopping flucytosine, and sometimes even with its continuation. Clinically apparent hepatotoxicity is very rare. Instances of acute liver injury and hepatic failure have been mentioned in clinical trials of flucytosine therapy, but few details were provided and no convincing case reports of acute hepatic injury with jaundice have been published.
Likelihood score: D (possible rare cause of clinically apparent liver injury.
Mechanism of Injury
The cause of serum aminotransferase elevations from flucytosine is unknown. While most flucytosine is excreted unchanged in the urine, a small proportion may be metabolized to fluorouracil, which may which may account for its bone marrow and liver toxicity in high doses.
Outcome and Management
The severity of the liver injury ranges from mild and transient enzyme elevations to clinically apparent liver injury in very rare cases. Typically, serum aminotransferase abnormalities improve rapidly upon discontinuation. Flucytosine has not been definitively implicated in cases of acute liver failure, chronic hepatitis or chronic vanishing bile duct syndrome. Studies of rechallenge have not been reported.
Drug Class: Antifungal Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Flucytosine – Generic, Ancobon®
DRUG CLASS
Antifungal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
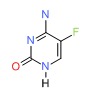
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Flucytosine | 2022-85-7 | C4-H4-F-N3-O |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 02 February 2018
- Zimmerman HJ. Antifungal agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 609-11.(Expert review of hepatotoxicity of antifungal agents published in 1999; mentions that transient serum enzyme elevations occur in 5-10% of patients treated with flucytosine, and a single case report of severe hepatitis attributed to flucytosine has been published).
- Moseley RH. Antibacterial and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 473.(Review of hepatotoxicity of antifungal agents mentions that asymptomatic liver chemistry abnormalities occur in up to 41% of patients, and severe liver injury has been reported in two patients).
- Bennett JE. Antimicrobial agents: antifungal agents. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1571-92.(Textbook of pharmacology and therapeutics).
- Record CO, Skinner JM, Sleight P, Speller DCE. Candida endocarditis treated with 5-fluorocytosine. Br Med J 1971; 1: 262-4. [PMC free article: PMC1794961] [PubMed: 5212581](3 patients with candida endocarditis were treated with flucytosine and amphotericin B, one recovered, two died, both with patchy hepatic necrosis on autopsy, but without major aminotransferase elevations or jaundice).
- Steer PL, Marks MI, Klite PD. 5-fluorocytosine oral antifungal compound: a report on clinical and laboratory experience. Ann Intern Med 1972; 76: 15-22. [PubMed: 5063168](Experience in treating 17 patients with invasive fungal infections with flucytosine; two patients developed ALT elevations [3-5 times normal], one resolving with dose reduction and one with discontinuation; both rapidly).
- Harder EJ, Hermans PE. Treatment of fungal infections with flucytosine. Arch Intern Med 1975; 135: 231-7. [PubMed: 1096840](Experience in treating 20 patients with invasive fungal infections with flucytosine alone or with amphotericin B; 6 patients [30%] had “slight” elevations in Alk P and 2 in AST levels, which became normal when flucytosine was stopped).
- Diasio RB, Lakings DE, Bennett JE. Evidence for conversion of 5-fluorocytosine to 5-fluorouracil in humans: possible factor in 5-fluorocytosine clinical toxicity. Antimicrob Agents Chemother 1978; 14: 903-8. [PMC free article: PMC352577] [PubMed: 742878](Significant levels of 5-fluorouracil was found in 41 serum samples from patients receiving flucytosine for fungal infections; levels similar to those in patients on cancer chemotherapy).
- Bennett JE, Dismukes WE, Duma RJ, Medoff G, Sande MA, Gallis H, Leonard J, et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N Engl J Med 1979; 301: 126-31. [PubMed: 449951](Trial of amphotericin alone vs its combination with flucytosine in 50 patients with cryptococcal meningitis; “no abnormalities of serum alkaline phosphatase or transaminase were caused by either regimen”).
- Stamm AM, Diasio RB, Dismukes WE, Cloud GA, Bowles CA, Karam GH, Espinel-Ingroff A. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Toxicity of amphotericin B plus flucytosine in 194 patients with cryptococcal meningitis. Am J Med 1987; 83: 236-42. [PubMed: 3303926](Controlled trial of 4 vs 6 weeks of amphotericin B and flucytosine in 194 patients with cryptococcal meningitis; elevated liver enzymes arose during first 4 weeks in 13 [6.2%] patients, and a 77 year old man with Crohn's disease developed signs and symptoms of hepatitis while on azathioprine as well as flucytosine, dying of acute liver failure one month later; authors attributed liver injury to flucytosine).
- Vermes A, van Der Sijs H, Guchelaar HJ. Flucytosine: correlation between toxicity and pharmacokinetic parameters. Chemotherapy 2000; 46: 86-94. [PubMed: 10671757](No correlation was found between pharmacokinetic parameters and likelihood of ALT or Alk P elevations in 53 patients treated with flucytosine and monitored for side effects; in contrast, there was a correlation with thrombocytopenia).
- Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother 2000; 46: 171-9. [PubMed: 10933638](Review of structure, mechanism of action, pharmacokinetics, efficacy and toxicity of flucytosine; reported rates of ALT elevations range from 0-41%, usually reversed with dose reduction and effect may be dose related).
- Song JC, Deresinski S. Hepatotoxicity of antifungal agents. Curr Opin Investig Drugs 2005; 6: 170-7. [PubMed: 15751740](Extensive review of hepatotoxicity from antifungals; wide range of patterns of injury have been reported, typically arising within first month of therapy, may be more common with high plasma levels which can be monitored).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, 8 cases were attributed to antifungal agents, but no cases were attributed to flucytosine).
- Antifungal drugs. Treat Guidel Med Lett 2009; 7: 95-102. [PubMed: 19940816](Concise summary of therapy of fungal infections with recommendations on agents, dosage and duration of treatment and safety; mentions that fluctosine has potentially lethal, dose related bone marrow toxicity; no mention of hepatotoxicity).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, 6 of which were attributed to antifungal agents, but none to flucytosine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 14 cases [1.6%] were attributed to antifungal agents including 7 due to terbinafine, 6 triazoles, 1 micafungin but none to flucytosine).
- Kyriakidis I, Tragiannidis A, Munchen S, Groll AH. Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf 2017; 16: 149-65. [PubMed: 27927037](Review of the hepatotoxicity of antifungal agents states that all antifungal agents may cause hepatic toxicity, flucytosine being linked to serum enzyme elevations during therapy in a variable proportion of patients and to rare instances of "severe liver necrosis").
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- In vitro pharmacodynamic characteristics of flucytosine determined by time-kill methods.[Diagn Microbiol Infect Dis. 2000]In vitro pharmacodynamic characteristics of flucytosine determined by time-kill methods.Lewis RE, Klepser ME, Pfaller MA. Diagn Microbiol Infect Dis. 2000 Feb; 36(2):101-5.
- Adaptation to Fluconazole via Aneuploidy Enables Cross-Adaptation to Amphotericin B and Flucytosine in Cryptococcus neoformans.[Microbiol Spectr. 2021]Adaptation to Fluconazole via Aneuploidy Enables Cross-Adaptation to Amphotericin B and Flucytosine in Cryptococcus neoformans.Yang F, Gritsenko V, Lu H, Zhen C, Gao L, Berman J, Jiang YY. Microbiol Spectr. 2021 Oct 31; 9(2):e0072321. Epub 2021 Sep 29.
- Review Flucytosine and its clinical usage.[Ther Adv Infect Dis. 2023]Review Flucytosine and its clinical usage.Sigera LSM, Denning DW. Ther Adv Infect Dis. 2023 Jan-Dec; 10:20499361231161387. Epub 2023 Apr 5.
- Flucytosine primary resistance in Candida species and Cryptococcus neoformans.[Eur J Clin Microbiol Infect Di...]Flucytosine primary resistance in Candida species and Cryptococcus neoformans.Cuenca-Estrella M, Díaz-Guerra TM, Mellado E, Rodríguez-Tudela JL. Eur J Clin Microbiol Infect Dis. 2001 Apr; 20(4):276-9.
- Review Posaconazole.[LiverTox: Clinical and Researc...]Review Posaconazole.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Flucytosine - LiverToxFlucytosine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...