NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Thioguanine (also referred to as 6-thioguanine and as tioguanine) is a purine analogue that is used in the therapy of acute and chronic myelogenous leukemias. Thioguanine therapy is associated with minor, usually transient and asymptomatic elevations in serum aminotransferase levels and has also been linked to rare instances of cholestatic acute liver injury and to chronic liver injury, resulting in portal hypertension due to nodular regenerative hyperplasia.
Background
Thioguanine (thye" oh gwa' neen) is a thiopurine, a purine analogue and antimetabolite. It is a derivative of mercaptopurine (2-amino-6-mercaptopurine) and, like its parent molecule, inhibits purine metabolism, thus blocking DNA, RNA and subsequent protein synthesis. Thioguanine also has antiinflammatory activity. Thioguanine was approved for use in the United States in 1966 and is commonly used in the therapy of acute and chronic myelogenous (nonlymphocytic) leukemias. Thioguanine has also been used off-label to treat autoimmune diseases as a steroid sparing agent. Thioguanine is available generically and under the brand name of Tabloid as tablets of 40 mg. The usual dose is 1 to 3 mg per kilogram or 40 to 120 mg daily and it is typically given long term. Common side effects include nausea, abdominal upset, rash, aphthous ulcers and dose related bone marrow suppression.
Hepatotoxicity
As with other thiopurines, thioguanine has been associated with several forms of hepatotoxicity, including mild, transient and asymptomatic rises in serum aminotransferase levels, an acute hepatic injury developing during the first year of starting therapy, and a chronic hepatic injury marked by variable degrees of peliosis hepatis, veno-occlusive disease and/or nodular regenerative hyperplasia. Chronic injury typically arises 1 to 5 years after starting thioguanine and can present insidiously with signs and symptoms of portal hypertension.
Mild serum aminotransferase elevations can occur during thioguanine therapy, particularly with high doses during the first 12 weeks of therapy. These elevations are generally asymptomatic, benign and self-limited, resolving rapidly either with stopping therapy, decreasing the dose and often even with continuing treatment without modifications. ALT elevations during thioguanine therapy may be due to a direct toxic effect of the drug; ALT elevations as well as myelotoxicity have been linked to higher levels of methyl-mercaptopurine, a product of one of the metabolic pathways of thioguanine metabolism.
The acute hepatic injury due to thioguanine usually presents with fatigue and jaundice and with a mixed hepatocellular-cholestatic pattern of serum enzyme elevations after 2 to 12 months of starting therapy. Rash, fever and eosinophilia are uncommon and autoantibodies are generally not found. Liver biopsy typically shows intrahepatic cholestasis with focal hepatocellular necrosis and scant inflammation. The liver injury usually resolves rapidly on stopping, but prolonged cholestasis has been reported and some cases have been fatal. This form of hepatotoxicity appears to be idiosyncratic and a class effect of the thiopurines, although more typical of azathioprine than thioguanine or mercaptopurine.
The chronic thioguanine hepatotoxicity typically presents with fatigue and signs and symptoms of portal hypertension with mild liver enzyme abnormalities and minimal jaundice arising 6 months to many years after starting thioguanine. Liver biopsy shows nodular regenerative hyperplasia and varying amounts of sinusoidal dilation and central vein injury. This syndrome can progress to hepatic failure, particularly if thioguanine is continued, but improvement on stopping therapy is typical. The onset of this syndrome may be acute with abdominal pain and ascites, in which situation liver biopsy usually shows sinusoidal dilation, central congestion and injury to sinusoidal endothelial cells suggestive of veno-occlusive disease, which is currently referred to as sinusoidal obstructive syndrome. Typically, serum aminotransferase levels and alkaline phosphatase levels are minimally elevated, even in the presence of hyperbilirubinemia and other manifestations of hepatic dysfunction and portal hypertension. Many cases of nodular regenerative hyperplasia due to the thiopurines present initially with thrombocytopenia of unknown cause, and a gradual decrease in platelet count is probably the most reliable marker for the development of non-cirrhotic portal hypertension. Among the thiopurines, this syndrome is more frequent with thioguanine than azathioprine, and appears to be least frequent with mercaptopurine.
Finally, long term therapy with thiopurines has been implicated in leading to the development of malignancies, including hepatocellular carcinoma (HCC) and hepatosplenic T cell lymphoma (HSTCL). Both of these complications are rare, but have been reported in several dozen case reports and small case series, most frequently with azathioprine. In neither instance, has the role of thiopurine therapy in causing the malignacies been proven, and similar cases have been described in patients with autoimmune conditions or after solid organ transplantation who have not received thiopurines. Hepatocellular carcinoma typically arises after years of thiopurine therapy and in the absence of accompanying liver disease (although sometimes with focal hepatic glycogenosis). The HCC is most frequently found on an imaging study done of an unrelated condition. The prognosis is more favorable than that of HCC associated with cirrhosis. Hepatosplenic T cell lymphoma has been reported largely among young men with inflammatory bowel disease and long term immunosuppression with a thiopurine with or without anti-tumor necrosis factor therapy. The typical presentation is with fatigue, fever, hepatosplenomegaly and pancytopenia. The diagnosis is made by bone marrow or liver biopsy showing marked infiltration with malignant T cells. HSTCL is poorly responsive to antineoplastic therapy and has a high mortality rate.
Likelihood score: A (well known cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which thioguanine causes idiosyncratic acute liver injury is not known, but is likely due to an immunological response to a metabolic byproduct of its metabolism. Thioguanine undergoes extensive hepatic metabolism to 6-mercaptopurine and thereafter to other thiopurines via three different pathways. Patients with deficiency in thiopurine methyltransferase which mediates one of these metabolic pathways have a higher rate of complications of thioguanine use, particularly bone marrow suppression. but do not appear to be at higher rise of acute cholestasis or nodular regeneration. The cause of the nodular regenerative hyperplasia that develops after long term thioguanine therapy is not well defined, but it appears to be due to injury to endothelial cells that causes variable degrees of venous outflow obstruction or vascular damage that promotes the nodular transformation. This form of injury is more likely to be a direct toxic effect of the antimetabolite.
Outcome and Management
The serum aminotransferase elevations that occur during thioguanine therapy may improve spontaneously or with dose reduction and generally resolve rapidly with discontinuation. In patients who have aberrant metabolism of thiopurines to 6-methylmercaptopurine (6-MMP) as shown by elevated plasma levels, lowering the dose of thiopurine and adding allopurinol (100 mg daily) may lower 6-MMP levels, reverse aminotransferase elevations while maintaining 6-thioguanine (6-TGN) levels and clinical response. Both the acute cholestasis and the chronic nodular regeneration caused by thioguanine improve upon stopping the medication, but instances of progression to hepatic failure despite discontinuation of thioguanine have been reported with both syndromes. Rechallenge with thioguanine usually results in recurrence of the injury (within days to weeks) and should be avoided. Some patients have tolerated switching therapy to mercaptopurine or azathioprine, but substitution with a structurally unrelated antimetabolite may be more appropriate.
Drug Class: Antineoplastic Agents, Antimetabolites
Other Drugs in the Subclass, Purine Analogues: Azathioprine, Cladribine, Clofarabine, Fludarabine, Mercaptopurine, Nelarabine, Pentostatin
See also: Transplant Drugs
Other Drugs in the Subclass, Purine Analogues/Thiopurines: Azathioprine, Mercaptopurine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Thioguanine – Generic, Tabloid®
DRUG CLASS
Antineoplastic Agents
Antirheumatic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Thioguanine | 154-42-7 | C5-H5-N5-S |
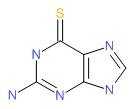 |
ANNOTATED BIBLIOGRAPHY
References updated: 17 August 2017
- Zimmerman HJ. Antipurines. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 687-9.(Expert review of hepatotoxicity of thiopurines published in 1999).
- DeLeve LD. Thiopurines. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2011, pp. 555-6.(Review of hepatotoxicity of thiopurines).
- Chabner BA, Bertino J, Cleary J, Ortiz T, Lane A, Supko JG, Ryan D. Purine analogues. Cytotoxic agents. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1701-2.(Textbook of pharmacology and therapeutics).
- Sparberg M, Simon N, Del Greco F. Intrahepatic cholestasis due to azathioprine. Gastroenterology 1969; 57: 439-41. [PubMed: 4951148](44 year old man developed jaundice 13 months after renal transplant [bilirubin 21 mg/dL, ALT 94 U/L, Alk P normal], biopsy showing cholestasis without hepatocyte necrosis; patient died after withdrawal of azathioprine and increase in corticosteroids).
- Griner PF, Elbadawi A, Packman CH. Veno-occlusive disease of the liver after chemotherapy of acute leukemia. Report of two cases. Ann Intern Med 1976; 85: 578-82. [PubMed: 1068643](Two men with leukemia, ages 17 and 72 years, developed abdominal pain, ascites and progressive liver failure 3-4 months after starting cyclic cytotoxic therapy with multiple agents including thioguanine [initial bilirubin 1.3 and 2.5 mg/dL, AST 348 and 48 U/L, Alk P 92 and 276 U/L]; autopsies showed acute sinusoidal obstruction syndrome [veno-occlusive disease]).
- Nataf C, Feldmann G, Lebrec D, Degott C, Descamps JM, Rueff B, Benhamou JP. Idiopathic portal hypertension (perisinusoidal fibrosis) after renal transplantation. Gut 1979; 20: 531-7. [PMC free article: PMC1412455] [PubMed: 381128](Two cases of young men developing esophageal varices 1.5 and 7 years after renal transplantation and azathioprine therapy with normal liver tests and liver biopsy, but perisinusoidal fibrosis found on electron microscopy, seemingly improving once azathioprine was stopped).
- Ware AJ, Luby JP, Hollinger B, Eigenbrodt EH, Cuthbert JA, Atkins CR, Shorey J, et al. Etiology of liver disease in renal-transplant patients. Ann Intern Med 1979; 91: 364-71. [PubMed: 224742](Analysis of 72 episodes of liver disease occurring in 62 of 120 renal transplant recipients; 4 episodes of acute cholestasis were attributed to azathioprine, resolving with stopping or dose reduction; azathioprine did not seem to account for cases of chronic liver injury, responses to stopping being equivocal).
- Stromeyer FU, Ishak KG. Nodular transformation(nodular regenerative hyperplasia) of the liver. A clinicopathological study of 30 cases. Hum Pathol 1981; 1: 60-71. [PubMed: 7203455](30 cases of nodular regenerative hyperplasia from the files of the Armed Forces Institute of Pathology; ages 14-80 years, half males, 18 discovered incidentally, 84% splenomegaly, 58% ascites, half died, 3 with liver failure, mild elevations in AST and Alk P levels were typical; 6 had rheumatologic disorder, 7 hematologic disease; 10 on corticosteroids, several on azathioprine [but no mention of thioguanine or mercaptopurine]; detailed description of histological changes).
- Milligan DW, Thein SL, Roberts BE. Secondary treatment of polycytemia rubra vera with 6-thioguanine. Cancer 1982; 50: 836-9. [PubMed: 7093923](Among 29 patients with polycytemia vera treated with thioguanine for 6-66 months, 89% had a clinical response; 3 had transient serum enzyme elevations, 1 developed cholestatic jaundice 12 months after starting which resolved on stopping).
- Weitz H, Gokel SM, Loeschke K, Possinger K, Eder M. Venoocclusive disease of the liver in patients receiving immunosuppressive therapy. Virchows Arch [1] 1982; 395: 245-56. [PubMed: 7051531](Four cases of sinusoidal obstruction syndrome in 34-41 year old men who developed mild jaundice 9-21 months after renal transplantation [bilirubin 2.8-3.7 mg/dL, ALT 17-60 U/L, Alk P 108-673 U/L], ultimately with fatal outcomes).
- Gill RA, Onstad GR, Cardamone JM, Maneval DC, Sumner HW. Hepatic veno-occlusive disease caused by 6-thioguanine. Ann Intern Med 1982; 96: 58-60. [PubMed: 7053705](23 year old man with acute leukemia developed anorexia and jaundice 2 months after induction of remission and while on maintenance methotrexate [bilirubin 1.3 mg/dL, AST 3 times ULN], recovering upon switching to thioguanine, but developing symptoms and signs of sinusoidal obstruction syndrome one year later).
- Krivoy N, Raz R, Carter A, Alroy G. Reversible hepatic veno-occlusive disease and 6-thioguanine. Ann Intern Med 1982; 96: 788. [PubMed: 7091946](Letter in response to Gill [1982] reporting another case of sinusoidal obstruction syndrome in a 46 year old man with leukemia being treated with methotrexate and thioguanine for 3 months [initial bilirubin 1.4 mg/dL, Alk P 5 times ULN, Alk P 997 U/L, platelets 94,000/μL], resolving with stopping chemotherapy).
- Satti MB, Weinbren K, Gordon-Smith EC. 6-thioguanine as a cause of toxic veno-occlusive disease of the liver. J Clin Pathol 1982; 3: 1086-91. [PMC free article: PMC497888] [PubMed: 6957419](Two patients with leukemia developed sinusoidal obstruction syndrome after chemotherapy with multiple agents including thioguanine, but also busulfan, cytosine arabinoside, daunorubicin and total body irradiation).
- D'Cruz CA, Wimmer RS, Harcke HT, Huff DS, Naiman JL. Veno-occlusive disease of the liver in children following chemotherapy for acute myelocytic leukemia. Cancer 1983; 52: 1803-7. [PubMed: 6578867](Three children [2 girls and 1 boy, ages 4, 8 and 14] developed sinusoidal obstruction syndrome 5-6 months after starting chemotherapy for leukemia presenting with abdominal pain and hepatomegaly [bilirubin 1.2-2.0 mg/dL, ALT and Alk P normal], improving on decreasing dose of chemotherapy).
- DePinho RA, Goldberg CS, Lefkowitch JH. Azathioprine and the liver: evidence favoring idiosyncratic, mixed cholestatic-hepatocellular injury in man. Gastroenterology 1984; 86: 162-5. [PubMed: 6689657](22 year old man with lupus erythematosus developed jaundice 3 weeks after starting azathioprine [peak direct bilirubin ~4.9 mg/dL, ALT ~710 U/L, Alk P ~560 U/L], resolving within 6 weeks of stopping; biopsy showed intrahepatic cholestasis with scant inflammation and necrosis: Azathioprine Case 1).
- Eisenhauer T, Hartmann H, Rumpf KW, Helmchen U, Scheler F, Creutzfeldt W. Favorable outcome of hepatic veno-occlusive disease in a renal transplant patient receiving azathioprine, treated by portacaval shunt: report of a case and review of the literature. Digestion 1984; 30: 185-90. [PubMed: 6389237](45 year old man developed ascites and abdominal pain 2 years after renal transplant while on azathioprine, biopsy showing veno-occlusive disease and ascites, responding to stopping azathioprine and portacaval shunt).
- Gerlag PG, Labatty S, Driessen WM, Deckers PFL, Van Hooff JP, Schroder E, Assmann KM, et al. Hepatic sinusoidal dilatation with portal hypertension during azathioprine treatment after kidney transplantation. J Hepatol 1986; 1: 339-48. [PubMed: 3902952](3 patients developed evidence of portal hypertension 16-24 months after renal transplant on azathioprine therapy without portal fibrosis, improvement on stopping but residual changes found during follow up).
- Read AE, Wiesner RH, La Brecque DR, Tifft JG, Mullen KD, Sheer RL, Petrelle M, et al. Hepatic veno-occlusive disease associated with renal transplantation and azathioprine therapy. Ann Intern Med 1986; 104: 651-6. [PubMed: 3008617](Four patients with veno-occlusive disease and signs of portal hypertension 0.5-9 years after renal transplantation while on azathioprine; one improved, 3 had progressive problems with portal hypertension and hepatic failure).
- Katzka DA, Saul SH, Jorkasky D, Sigal H, Reynolds JC, Soloway RD. Azathioprine and hepatic venoocclusive disease in renal transplant patients. Gastroenterology 1986; 90: 446-54. [PubMed: 3510146](3 men, ages 37-59 years, developed liver test abnormalities [bilirubin 1.5-3.5 mg/dL, ALT 44-86 U/L, Alk P 505-648 U/L] 12-32 months after starting azathioprine, 2 dying and one surviving with improvement after stopping azathioprine; all had nodular regenerative hyperplasia and sinusoidal obstructive changes on biopsy or autopsy).
- Adler M, Delhaye M, Deprez C, Hardy N, Gelin M, DePauw L, Vererstraeten P, et al. Hepatic vascular disease after kidney transplantation: report of two cases and review of the literature. Nephrol Dialysis Transplant 1987; 2: 183-8. [PubMed: 3114679](Two men, ages 31 and 40 years, developed portal hypertension due to azathioprine 9 and 4 years after kidney transplant, presenting with fatigue and abdominal distension with mild jaundice and minimal elevations in Alk P and ALT, biopsy showed changes of peliosis and sinusoidal obstruction syndrome; subsequently both had hepatic decompensation and one died).
- Fonseca V, Havard CW. Portal hypertension secondary to azathioprine in myasthenia gravis. Postgrad med J 1988; 64: 950-2. [PMC free article: PMC2429092] [PubMed: 3256814](52 year old man with myasthenia was found to have splenomegaly 3 years after starting azathioprine [150 mg/day] [bilirubin 4.1 mg/dL, AST 40 U/L, Alk P 107 U/L, platelets 103,000/μL], biopsy showing nodular regenerative hyperplasia with improvement in liver tests [bilirubin 1.2 mg/dL] upon stopping).
- Buffet C, Cantarovitch M, Pelletier G, Fabre M, Martin E, Charpentier B, Etienne JP, et al. Three cases of nodular regenerative hyperplasia of the liver following renal transplantation. Nephrol Dial Transplant 1988; 3: 327-30. [PubMed: 3140108](Three men, ages 22, 29 and 50 years, developed nodular regenerative hyperplasia with hepato-splenomegaly 24-30 months after renal transplant while on azathioprine, two had peliosis and one sinusoidal obstruction syndrome; minimal liver test abnormalities in two; jaundice in the third who had a fatal outcome [initial bilirubin 5.8 mg/dL, AST 35 U/L, Alk P 80 U/L]).
- Jones MC, Best PV, Catto GRD. Is nodular regenerative hyperplasia of the liver associated with azathioprine therapy after renal transplantation. Nephrol Dial Transplant 1988; 3: 330-3. [PubMed: 3140109](33 year old man developed minor elevations in liver tests starting 15 months after renal transplantation while on azathioprine [bilirubin 1.0 mg/dL, AST 42 U/L, Alk P 185 U/L], later developing hepatomegaly and ascites; biopsy showed nodular regenerative hyperplasia).
- Larrey D, Freneaux E, Berson A, Babany G, DeGott C, Valla D, Pessayre D, et al. Peliosis hepatic induced by 6-thioguanine administration. Gut 1988; 29: 1265-9. [PMC free article: PMC1434359] [PubMed: 3198003](63 year old woman developed jaundice 2 months after chemotherapy for acute leukemia while on thioguanine and cytosine arabinoside [bilirubin 5.6 mg/dL, ALT 142 U/L, Alk P 335 U/L], biopsy showed congestion and endothelial injury; slow improvement on stopping thioguanine).
- Lemley DE, Delacy LM, Seeff LB, Ishak KG, Nashel DJ. Azathioprine induced hepatic veno-occlusive disease in rheumatoid arthritis. Ann Rheum Dis 1989; 48: 342-6. [PMC free article: PMC1003755] [PubMed: 2712618](59 year old man with rheumatoid arthritis developed abdominal pain 3 months after starting azathioprine with bilirubin 0.2 mg/dL, with minimal elevations in ALT and Alk P; azathioprine was continued and signs of portal hypertension arose 4 months later, biopsy showing sinusoidal obstruction syndrome).
- Ramalho HJ, Terra EG, Cartapatti E, Barberato JB, Alves VA, Gayotto LC, Abbud-Filho M. Hepatotoxicity of azathioprine in renal transplant recipients. Transplant Proc 1989; 21 (1 Pt 2): 1716-7. [PubMed: 2652562](2 women and 2 men, ages 28-49 years, developed cholestasis 2-18 months after renal transplant while on azathioprine [bilirubin 9.3-44 mg/dL, ALT 62-842 U/L, Alk P 1-3 times ULN], improving on stopping and reappearing within weeks on restarting azathioprine).
- Horsmans Y, Rahier J, Geubel AP. Reversible cholestasis with bile duct injury following azathioprine therapy. A case report. Liver 1991; 11: 89-93. [PubMed: 2051906](67 year old man with polymyositis developed jaundice 3 months after starting azathioprine [bilirubin ~5.0 mg/dL, ALT ~120 U/L, Alk P 550 U/L], liver biopsy showing intrahepatic cholestasis and bile duct injury; resolving within 2 months of stopping).
- Sterneck M, Wiesner R, Ascher N, Roberts J, Ferrell L, Ludwig J, Lake J. Azathioprine hepatotoxicity after liver transplantation. Hepatology 1991; 14: 806-10. [PubMed: 1937385](Two patients, 58 year old woman and 41 year old man, developed jaundice 2 and 8 weeks after liver transplantation [bilirubin 33.8 and 7.1 mg/dL, AST 456 and 2965 U/L, Alk P not given and 349 U/L], liver biopsies showing centrilobular congestion, sinusoidal dilation and hemorrhagic necrosis suggesting vascular outflow obstruction, both improved on stopping azathioprine, one worsened on rechallenge).
- Shepherd PC, Fooks J, Gray R, Allan NC. Thioguanine used in maintenance therapy of chronic myeloid leukaemia causes non-cirrhotic portal hypertension. Results from MRC CML. II. Trial comparing busulphan with busulphan and thioguanine. Br J Haematol 1991; 79: 185-92. [PubMed: 1958475](Among 674 patients with chronic leukemia, 18 of 337 treated with busulphan and thioguanine developed portal hypertension after 1-8 years [median 2 years] compared to none of 338 given busulphan alone, whereas liver test abnormalities [~50%] and jaundice [~3%] occurred in similar proportions of both groups).
- Mion F, Napoleon B, Berger F, Chevallier M, Bonvoisin S, Descos L. Azathioprine induced liver disease: nodular regenerative hyperplasia of the liver and perivenous fibrosis in a patient treated for multiple sclerosis. Gut 1991; 32: 715-7. [PMC free article: PMC1378897] [PubMed: 2060883](37 year old man with multiple sclerosis developed variceal hemorrhage 7 years after starting azathioprine [100-150 mg/day] with biopsy showing nodular regeneration and perivascular fibrosis without inflammation).
- Kao NL, Rosenblate HJ. 6-thioguanine therapy for psoriasis causing toxic hepatic venoocclusive disease. J Am Acad Dermatol 1993; 28: 1017-8. [PubMed: 8496447](33 year old man with psoriasis developed fever and jaundice [bilirubin 8.6 mg/dL, ALT 366 U/L, Alk P 168 U/L, platelets 37,000/ μL] after starting thioguanine and increasing the dose; biopsy showed sinusoidal obstruction syndrome and toxic hepatitis, improving slowly over next 6 months after medications were stopped).
- Gane E, Portmann B, Saxena R, Wong P, Ramage J, Williams R. Nodular regenerative hyperplasia of the liver graft after liver transplantation. Hepatology. 1994; 20: 88-94. [PubMed: 8020909](Nine cases of biopsy proven nodular regenerative hyperplasia in liver transplant recipients arising after 0.5-12 years; 6 presenting with ascites or varices and 3 without symptoms [bilirubin 0.5-5.1 mg/dL, AST 36-192 U/L, Alk P 159-1182 U/L], 5 improving on stopping azathioprine and 4 developing progressive hepatic failure; several had earlier biopsies showing venous outflow obstruction).
- Dhillon AP, Burroughs AK, Hudson M, Shah N, Rolles K, Scheuer PJ. Hepatic venular stenosis after orthotopic liver transplantation. Hepatology 1994; 19: 106-11. [PubMed: 8276346](Retrospective review of 49 liver biopsies taken after liver transplantation identified 7 patients with venous outflow obstruction usually within 30 days of transplant, associated with endothelitis, only 5 on azathioprine suggesting that changes were due to rejection rather than drug).
- Stetter M, Schmidl M, Krapf R. Azathioprine hypersensitivity mimicking Goodpasture's syndrome. Am J Kidney Dis 1994; 23: 874-7. PubMed Citation. [PubMed: 8203372](Patient with renal transplant developed fever, arthralgias, diarrhea and pulmonary infiltrates within 7 days of starting azathioprine on three occasions, but later tolerated mercaptopurine suggesting hypersensitivity to nitroimidazole component of azathioprine).
- Kowdley KV, Keeffe EB. Hepatotoxicity of transplant immunosuppressive agents. Gastroenterol Clin North Am 1995; 24: 991-1001. [PubMed: 8749908](Review of hepatotoxicity of agents used in solid organ transplantation including azathioprine, which causes a broad range of hepatic injuries including transient ALT elevations, cholestasis, sinusoidal dilatation, veno-occlusive disease, peliosis and nodular regenerative hyperplasia with portal hypertension).
- Romagnuolo J, Sadowski DC, Lalor E, Jewell L, Thomson AB. Cholestatic hepatocellular injury with azathioprine: a case report and review of the mechanisms of hepatotoxicity. Can J Gastroenterol 1998; 12: 479-83. [PubMed: 9812167](63 year old man with Crohn's disease developed jaundice 9 weeks after starting azathioprine [bilirubin 8.0 mg/dL, AST 65 U/L, Alk P 896 U/L], liver biopsy showed intrahepatic cholestasis, resolved with stopping).
- Schwab M, Schaffeler E, Marx C, Fischer C, Lang T, Behrens C, Gregor M, et al. Azathioprine therapy and adverse drug reactions in patients with inflammatory bowel disease: impact of thiopurine S-methyltransferase polymorphism. Pharmacogenetics 2002; 12: 429-36. [PubMed: 12172211](Among 93 adults treated with azathioprine, thiopurine S-methyl transferase [TPMT] activities were normal in those with gastrointestinal [n=9] and liver toxicities [n=3], but were low in those with hematological toxicities).
- Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, Martin P, et al. 6-thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology 2003; 125: 298-303. [PubMed: 12891528](Among 111 patients with inflammatory bowel disease treated with thioguanine at one institution, 27 [25%] developed abnormal liver tests [ALT 18-139 U/L; Alk P 35-221 U/L], correlating with higher methyl-metabolites of mercaptopurine and fall in platelet count [from 300,000 to 166,000/μL], nodular regeneration found in 76% of 17 patients biopsied with abnormal liver tests and 33% of nine with normal tests).
- Rulyak SJ, Saunders MD, Lee SD. Hepatotoxicity associated with 6-thioguanine therapy for Crohn's disease. J Clin Gastroenterol 2003; 36: 234-7. [PubMed: 12590235](27 year old with Crohn's disease developed asymptomatic rise in ALT to 191 U/L 2 weeks after starting thioguanine, ALT then rising to 803 U/L at 4 weeks with normal Alk P and minimal increase in bilirubin [1.5 mg/dL], resolving within 1 month of stopping).
- Stoneham S, Lennard L, Coen P, Lilleyman J, Saha V. Veno-occlusive disease in patients receiving thiopurines during maintenance therapy for childhood acute lymphoblastic leukaemia. Br J Haematol 2003; 123: 100-2. [PubMed: 14510948](Retrospective analysis of veno-occlusive disease among 99 children who received thioguanine or mercaptopurine for acute leukemia; 12 developed veno-occlusive disease all of whom received thioguanine and were male; no correlation with dose or thiopurine methyltransferase levels).
- Piel B, Vaidya S, Lancaster D, Taj M, Pritchard-Jones K. Chronic hepatotoxicity following 6-thioguanine therapy for childhood acute lymphoblastic leukaemia. Br J Haematol 2004; 125: 410-1; author reply 412. [PubMed: 15086428](Letter in response to Stoneham [2003] reporting veno-occlusive disease in 7 of 59 children receiving thioguanine [12%] with chronic liver injury in 7 [suspected to be nodular regenerative hyperplasia]; patients tolerated being switched to mercaptopurine).
- Geller SA, Dubinsky MC, Poordad FF, Vasiliauskas EA, Cohen AH, Abreu MT, Tran T, et al. Early hepatic nodular hyperplasia and submicroscopic fibrosis associated with 6-thioguanine therapy in inflammatory bowel disease. Am J Surg Pathol 2004; 28: 1204-11. [PubMed: 15316320](Early nodular regenerative hyperplasia found in 20 of 39 [53%] biopsies done in evaluation of abnormal liver tests in patients with inflammatory bowel disease receiving 6-thioguanine; often required reticulin staining to demonstrate).
- Kontorinis N, Agarwal K, Gondolesi G, Fiel MI, O'Rourke M, Schiano TD. Diagnosis of 6 mercaptopurine hepatotoxicity post liver transplantation utilizing metabolite assays. Am J Transplant 2004; 4: 1539-42. [PubMed: 15307844](Two women, ages 46 and 53 years, with liver transplants developed jaundice 5 and 3 months after starting mercaptopurine [bilirubin 11.7 and 8.0 mg/dL, ALT 108 and 392 U/L, Alk P 464 and 395 U/L], resolving within 1-2 months of stopping; neither had TPMT deficiency).
- Nygaard U, Toft N, Schmiegelow K. Methylated metabolites of 6-mercaptopurine are associated with hepatotoxicity. Clin Pharmacol Ther 2004; 75: 274-81. [PubMed: 15060506](High frequency of ALT elevations occurred in children with acute leukemia treated with mercaptopurine, usually resolving with stopping or lowering the dose; ALT elevations were associated with higher doses and higher levels of methylated metabolites of mercaptopurine).
- Reuther LO, Vainer B, Sonne J, Larsen NE. Thiopurine methyltransferase(TPMT) genotype distribution in azathioprine-tolerant and -intolerant patients with various disorders. The impact of TPMT genotyping in predicting toxicity. Eur J Clin Pharmacol 2004; 59: 797-801. [PubMed: 14634700](Weak correlation found between azathioprine intolerance and TPMT mutations).
- de Boer NK, Mulder CJ, van Bodegraven AA. Myelotoxicity and hepatotoxicity during azathioprine therapy. Neth J Med 2005; 63: 444-6. [PubMed: 16397313](38 year old man with Crohn's disease was found to have abnormal liver tests and portal hypertension 3 years after starting azathioprine [bilirubin 2.1 mg/dL, ALT 53 U/L, Alk P 141 U/L], improving upon stopping).
- de Boer NK, De Graaf P, Wilhelm AJ, Mulder CJ, van Bodegraven AA. On the limitation of 6-tioguaninenucleotide monitoring during thioguanine treatment. Aliment Pharmacol Ther 2005; 22: 447-51. [PubMed: 16128683](Analysis of drug levels in 25 patients switched from azathioprine to thioguanine found no correlation with myelotoxicity; hepatotoxicity not mentioned).
- Bastida G, Nos P, Aguas M, Beltrán B, Rubín A, Dasí F, Ponce J. Incidence, risk factors and clinical course of thiopurine-induced liver injury in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2005; 22: 775-82. [PubMed: 16225485](Prospective analysis of 161 patients treated with azathioprine for inflammatory bowel disease; 16 [10%] developed elevations in liver tests at least twice normal after 2 days to 3 years [bilirubin normal, ALT 85-240 U/L, Alk P 48-526 U/L], resolving in all 16 patients including 11 who continued therapy).
- Heckmann JM, Lambson EM, Little F, Owen EP. Thiopurine methyltransferase(TPMT) heterozygosity and enzyme activity as predictive tests for the development of azathioprine-related adverse events. J Neurol Sci 2005; 231: 71-80. [PubMed: 15792824](7 of 129 neurological patients on azathioprine developed hepatotoxicity; testing for TPMT activity or mutations had low predictive value).
- Sparrow MP, Hande SA, Friedman S, Lim WC, Reddy SI, Cao D, Hanauer SB. Allopurinol safely and effectively optimizes thioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther 2005; 22: 441-6. [PubMed: 16128682](Adding allopurinol to azathioprine therapy led to higher thioguanine and methyl-mercaptopurine levels, thus allowing for reduction in azathioprine dose, and theoretically better efficacy, lower toxicity).
- Daniel F, Cadranel JF, Seksik P, Cazier A, Duong Van Huyen JP, Ziol M, Coutarel P, et al. Azathioprine induced nodular regenerative hyperplasia in IBD patients. Gastroenterol Clin Biol 2005; 29: 600-34. [PubMed: 15980758](Four men, ages 26-46 years, with inflammatory bowel disease developed nodular regenerative hyperplasia 6-12 months after starting azathioprine, presenting with liver test abnormalities and decrease in platelet counts, improving with stopping including slight increase in platelet count: Azathioprine Case 3).
- Broxson EH, Dole M, Wong R, Laya BF, Stork L. Portal hypertension develops in a subset of children with standard risk acute lymphoblastic leukemia treated with oral 6-thioguanine during maintenance therapy. Pediatr Blood Cancer 2005; 44: 226-31. [PubMed: 15503293](Among 12 children treated with thioguanine long term, 4 developed portal hypertension and another had transient veno-occlusive disease, usually presenting with normal liver tests, but persistent thrombocytopenia and splenomegaly with varices on imaging or endoscopy).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology 2005; 129: 512-21. [PubMed: 16083708](Among 446 cases of drug induced liver injury collected in Spain between 1984-2004, azathioprine accounted for 6 cases, ranking 14th; injury usually cholestatic, no fatalities; thioguanine not listed).
- De Bruyne R, Portmann B, Samyn M, Bansal S, Knisely A, Mieli-Vergani G, Dhawan A. Chronic liver disease related to 6-thoguanine in children with acute lymphoblastic leukaemia. J Hepatol 2006; 44: 407-10. [PubMed: 16226335](Evaluation of 6 children with acute leukemia who developed sinusoidal obstructive syndrome during thioguanine therapy; disease progressed despite switching to mercaptopurine, biopsies showing nodular regeneration; platelet counts 74-181,000/ μL).
- Derijks LJ, Gilissen LP, de Boer NK, Mulder CJ. 6-Thioguanine-related hepatotoxicity in patients with inflammatory bowel disease: dose or level dependent? J Hepatol 2006; 44: 821-2. [PubMed: 16487623](Letter in response to De Bruyne [2006] suggesting that use of lower doses and monitoring for metabolites may help avoid hepatotoxicity).
- Ravikumara M, Hill FG, Wilson DC, Gillett PM, Thomas A, Brown R, Darbyshire PJ, et al. 6-Thioguanine-related chronic hepatotoxicity and variceal haemorrhage in children treated for acute lymphoblastic leukaemia - a dual-centre experience. J Pediatr Gastroenterol Nutr 2006; 42: 535-8. [PubMed: 16707977](Retrospective analysis of 75 children treated with maintenance thioguanine after chemotherapy for leukemia identified 10 [13%] with portal hypertension [2 with variceal hemorrhage], biopsy in 5 showed nodular regenerative hyperplasia, persistent splenomegaly and thrombocytopenia in most).
- Seiderer J, Zech CJ, Diebold J, Schoenberg SO, Brand S, Tillack C, Göke B, Ochsenkühn T. Nodular regenerative hyperplasia: a reversible entity associated with azathioprine therapy. Eur J Gastroenterol Hepatol 2006; 18: 553-5. [PubMed: 16607155](54 year old developed jaundice 5 months after starting azathioprine for Crohn disease [bilirubin 7.5 mg/dL, but normal serum enzymes]; biopsy showing nodular regenerative hyperplasia, improvement on stopping).
- Heneghan MA, Allan ML, Bornstein JD, Muir AJ, Tendler DA. Utility of thiopurine methyltransferase genotyping and phenotyping, and measurement of azathioprine metabolites in the management of patients with autoimmune hepatitis. J Hepatol 2006; 45: 584-91. [PubMed: 16876902](Among 86 patients with autoimmune hepatitis treated with azathioprine, TPMT mutations did not predict toxicities, one patient developed hepatotoxicity with rise in ALT to 3 times ULN that improved with dose reduction).
- Teml A, Schwab M, Hommes DW, Almer S, Lukas M, Feichtenschlager T, Florin T, et al. A systematic survey evaluating 6-thioguanine-related hepatotoxicity in patients with inflammatory bowel disease. Wien Klin Wochenschr 2007; 119: 519-26. [PubMed: 17943403](Among 296 patients with inflammatory bowel disease treated with thioguanine for 1 month to 4 years at 15 medical centers, liver enzyme elevations occurred in 43 [15%] and nodular regenerative hyperplasia was found in 16 of 60 patients undergoing liver biopsy; older age was the only risk factor identified).
- Shaye OA, Yadegari M, Abreu MT, Poordad F, Simon K, Martin P, Papadakis KA, et al. Hepatotoxicity of 6-mercaptopurine (6-MP) and azathioprine (AZA) in adult IBD patients. Am J Gastroenterol 2007; 102: 2488-94. [PubMed: 17764490](Among 173 adult patients with inflammatory bowel disease treated with azathioprine or mercaptopurine, 8 developed ALT elevations greater than twice ULN [bilirubin 0.3-2.4 mg/dL, ALT 58-434 U/L, Alk P 54-130 U/L], resolving with stopping or lowering dose; toxicity had weak association with higher methyl metabolites in serum).
- Gisbert JP, Luna M, González-Lama Y, Pousa ID, Velasco M, Moreno-Otero R, Maté J. Liver injury in inflammatory bowel disease: long-term follow-up study of 786 patients. Inflamm Bowel Dis 2007; 13: 1106-14. [PubMed: 17455203](Long term follow up of 138 patients treated with azathioprine or mercaptopurine; incidence of any abnormal liver test was 7.1% per year, values >twice ULN occurred in only 2.6% and often resolved spontaneously; 5 of 49 required discontinuation).
- Gisbert JP, González-Lama Y, Maté J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol 2007; 102: 1518-27. [PubMed: 17391318](Systematic review of the literature on hepatotoxicity of thiopurines in patients with inflammatory bowel disease; rates of hepatotoxicity have ranged from 0-13% [averaging 1.4% per year] depending upon definition of injury and degree of monitoring; these agents can also cause syndromes of acute hypersensitivity reactions, acute cholestasis and chronic injury of endothelial cell injury and nodular regenerative hyperplasia).
- Gilissen LP, Derijks LJ, Driessen A, Bos LP, Hooymans PM, Stockbrügger RW, Engels LG. Toxicity of 6-thioguanine: no hepatotoxicity in a series of IBD patients treated with long-term, low dose 6-thioguanine. Some evidence for dose or metabolite level dependent effects? Dig Liver Dis 2007; 39: 156-9. [PubMed: 17188950](Among 13 patients with inflammatory bowel disease treated with thioguanine for at least 2 years, none had evidence of nodular regeneration by either liver biopsy [n=11] or imaging studies [n=11]).
- de Boer NK, van Bodegraven AA, Jharap B, de Graaf P, Mulder CJ. Drug Insight: pharmacology and toxicity of thiopurine therapy in patients with IBD. Nat Clin Pract Gastroenterol Hepatol 2007; 4: 686-94. [PubMed: 18043678](Review of pharmacokinetics and metabolism of thiopurine and management of intolerance using genetic tests for TPMT and measurements of metabolites).
- Gardiner SJ, Gearry RB, Burt MJ, Ding SL, Barclay ML. Severe hepatotoxicity with high 6-methylmercaptopurine nucleotide concentrations after thiopurine dose escalation due to low 6-thioguanine nucleotides. Eur J Gastroenterol Hepatol 2008; 20: 1238-42. [PubMed: 18989148](3 patients with autoimmune disorders who developed hepatotoxicity 1-4 months after dose of mercaptopurine or azathioprine was increased [bilirubin 6.9, 37 and 21.2 mg/dL, ALT 195, 200 and 79 U/L, Alk P 123, 109 and 194 U/L]; two progressed to hepatic failure and died; methyl-metabolites of mercaptopurine were elevated in all 3).
- Leong RW, Gearry RB, Sparrow MP. Thiopurine hepatotoxicity in inflammatory bowel disease: the role for adding allopurinol. Expert Opin Drug Saf 2008; 7: 607-16. [PubMed: 18759713](Allopurinol can increase levels of mercaptopurine and allow for dose reductions as well as shift its metabolism towards the active [6-thioguanine nucleotides] and away from toxic metabolites [methyl-mercaptopurine ribonucleotide], but requires monitoring).
- Lees CW, Maan AK, Hansoti B, Satsangi J, Arnott DR. Tolerability and safety of mercaptopurine in azathioprine-intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther 2008; 27: 220-7. [PubMed: 17988235](Retrospective analysis of 61 patients with inflammatory bowel disease who were intolerant to azathioprine; 36 [59%] were able to tolerate mercaptopurine, including 3 of 9 with hepatotoxicity).
- Ehmsen L, Marko C, Breidert M. [Portal vein hypertension during azathioprine therapy in patients with Crohn's disease - a frequent phenomenon?]. Dtsch Med Wochenschr 2008; 133: 950-3. German. [PubMed: 18431703](45 year old man with Crohn's disease on azathioprine for 4 years developed variceal hemorrhage [bilirubin 4.1 mg/dL, normal ALT, platelets 84,000/μL] and was found to have nodular regenerative hyperplasia on liver biopsy, and subsequently improved on stopping azathioprine).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, 3 were attributed to mercaptopurine, but none to azathioprine or thioguanine).
- Rahhal RM, Bishop WP. Initial clinical experience with allopurinol-thiopurine combination therapy in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2008; 14: 1678-82. [PubMed: 18521913](13 patients were treated with allopurinol and dose reduction of azathioprine [by ~60%] with overall increase in 6-thioguanine and decrease in methyl-metabolites; ALT elevations improved in all).
- Ansari A, Elliott T, Baburajan B, Mayhead P, O'Donohue J, Chocair P, Sanderson J, et al. Long-term outcome of using allopurinol co-therapy as a strategy for overcoming thiopurine hepatotoxicity in treating inflammatory bowel disease. Aliment Pharmacol Ther 2008; 28: 734-41. [PubMed: 19145729](11 patients with inflammatory bowel disease who developed hepatotoxicity within 1-8 weeks of starting azathioprine [n=10] or mercaptopurine [n=1] were treated with allopurinol and low doses of the thiopurine; 9 tolerated long term therapy without recurrence).
- Xin HW, Xiong H, Wu XC, Li Q, Xiong L, Yu AR. Relationships between thiopurine S-methyltransferase polymorphism and azathioprine-related adverse drug reactions in Chinese renal transplant recipients. Eur J Clin Pharmacol 2009; 65: 249-55. [PubMed: 19048245](Association found between TPMP activity and hemato- but not hepatotoxicity; only 2 of 16 patients with hepatotoxicity were heterozygous for TPMT mutations, none were homozygous).
- Hindorf U, Johansson M, Eriksson A, Kvifors E, Almer SH. Mercaptopurine treatment should be considered in azathioprine intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther 2009; 29: 654-61. [PubMed: 19183142](Analysis of usefulness of measuring thiopurine metabolites in management of 238 patients with autoimmune hepatitis on azathioprine, finding it useful only in managing patients with a poor response; patients with hepatotoxicity were not able to tolerate switch to mercaptopurine).
- Murakami A, Tanaka Y, Ueda M, Nagano Y, Kunisaki R, Morimoto M, Enaka M, et al. Hepatocellular carcinoma occurring in a young Crohn's disease patient. Pathol Int 2009; 59: 492-6. [PubMed: 19563414](25 year old man with 12 year history of Crohn disease presented with hepatocellular carcinoma approximately one year after starting infliximab; no other risk factors identified; review of literature identified 7 cases in patients with Crohn's, mean age 20 years, all had received azathioprine and two infliximab).
- Moran G, Dillon J, Green J. Crohn's disease, hepatosplenic T-cell lymphoma and no biological therapy: are we barking up the wrong tree? Inflamm Bowel Dis. 2009; 15 (9): 1281-2. [PubMed: 19067412](Although fewer than 200 cases of hepatospenic T cell lymphoma [HSTCL] have been described in the literature, 23 have occurred in patients with inflammatory bowel disease, all of whom were on thiopurines and 16 also on anti-tumor necrosis factor antagonists [anti-TNF]; the authors describe a 23 year old man with ulcerative colitis treated with azathioprine for 7 years without anti-TNF who developed HSTCL).
- Beigel F, Jürgens M, Tillack C, Subklewe M, Mayr D, Göke B, Brand S, et al. Hepatosplenic T-cell lymphoma in a patient with Crohn's disease. Nat Rev Gastroenterol Hepatol 2009; 6: 433-6. [PubMed: 19575026](58 year old man with 35 year history of Crohn disease on azathioprine for 5 years developed HSTCL).
- Miura H, Kawaguchi T, Takazoe M, Kitamura S, Yamada H. Hepatocellular carcinoma and Crohn's disease: a case report and review. Intern Med 2009; 48: 815-9. [PubMed: 19443977](52 year old Japanese man with 36 year history of Crohn disease, but never treated with thiopurines presented with hepatic mass, found to be HCC with chronic hepatitis in non-tumorous liver tissue).
- Ochenrider MG, Patterson DJ, Aboulafia DM. Hepatosplenic T-cell lymphoma in a young man with Crohn's disease: case report and literature review. Clin Lymphoma Myeloma Leuk 2010; 10: 144-8. [PubMed: 20371449](18 year old man with 5 year history of Crohn disease treated with mercaptopurine developed HSTCL [bilirubin and ALT levels normal]).
- Bermejo F, López-Sanromán A, Algaba A, Van-Domselaar M, Gisbert JP, García-Garzón S, Garrido E, et al. Mercaptopurine rescue after azathioprine-induced liver injury in inflammatory bowel disease. Aliment Pharmacol Ther 2010; 31: 120-4. [PubMed: 19709096](Retrospective analysis of 31 patients with inflammatory bowel disease who were switched from azathioprine to mercaptopurine because of liver toxicity; in 87% mercaptopurine was tolerated without evidence of further toxicity).
- Leung Y, Sparrow MP, Schwartz M, Hanauer SB. Long term efficacy and safety of allopurinol and azathioprine or 6-mercaptopurine in patients with inflammatory bowel disease. J Crohns Colitis 2009; 3: 162-7. [PubMed: 21172265](Among 25 patients with inflammatory bowel disease switched from thiopurines to thioguanine and allopurinol for up to 2 years, none developed abnormal liver tests or thrombocytopenia).
- Takatsu N, Matsui T, Murakami Y, Ishihara H, Hisabe T, Nagahama T, Maki S, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol 2009; 24: 1258-64. [PubMed: 19682195](TPMT deficiency variants were rare [~2%] in Japanese and were not increased among those with azathioprine intolerance due to toxicity; 2 cases had hepatotoxicity).
- Andrejic J, Rojas-Balcazar J, Dennis M, Berkelhammer C. Azathioprine-induced hypersensitivity hepatitis: tolerance to 6-mercaptopurine. Inflamm Bowel Dis. 2010; 16: 1828-9. [PubMed: 20196148](50 year old man with ulcerative colitis developed jaundice 4 weeks after starting azathioprine [bilirubin 4.6 mg/dL, ALT 1077 U/L, Alk P 361 U/L], resolving upon stopping and not recurring when treated with mercaptopurine [for up to 6 years]).
- Ishida M, Naka S, Shiomi H, Tsujikawa T, Andoh A, Nakahara T, Saito Y, et al. Hepatocellular carcinoma occurring in a Crohn's disease patient. World J Gastroenterol 2010; 16: 3215-8. [PMC free article: PMC2896762] [PubMed: 20593510](37 year old Japanese man treated with azathioprine for 8 years developed liver cancer without cirrhosis but with glycogenosis present; review of 10 reported cases, all without cirrhosis).
- Bryant DL, Miles CJ, Gearry RB. Nodular regenerative hyperplasia of the liver secondary to azathioprine in a patient with inflammatory bowel disease. N Z Med J 2010; 123: 74-6. [PubMed: 20581898](54 year old man developed ascites and splenomegaly 18 months after starting azathioprine for Crohn disease, biopsy showing nodular regenerative hyperplasia, improving upon stopping).
- Wang HS, Gao YJ, Li J, Lu FJ, Miao H, Qian XW, Zhu XF. [Clinical characteristics of hepatic veno-occlusive disease in 6 children with hematologic neoplasm treated with 6-thioguanine]. Zhonghua Er Ke Za Zhi 2010; 48: 708-10. Chinese. [PubMed: 21092535](Abstract only: 6 children ages 3 to 5 years developed sinusoidal obstruction syndrome during therapy of leukemia with thioguanine in combination with other agents; diagnosis made clinically).
- Leal-Valdivieso C, Naves JE, Mañosa M, Zabana Y, Cabré E, Domènech E. [Portal hypertension in patients with inflammatory bowel disease]. Gastroenterol Hepatol 2010; 33: 297-302. Spanish. [PubMed: 20206413](Two patients, man and woman, ages 29 and 34 years, developed portal hypertension after thiopurine therapy for 6 and 10 years).
- López-Martín C, de la Fuente-Fernández E, Corbatón P, Sánchez MC, Gisbert JP. [Nodular regenerative hyperplasia: azathioprine-induced hepatotoxicity in a patient with Crohn's disease]. Gastroenterol Hepatol 2011; 34: 16-9. Spanish. [PubMed: 21168244](53 year old man developed abnormal liver tests after 5 years of azathioprine therapy [7 months after dose increase] for Crohn disease [bilirubin 2.2 mg/dL, ALT 61 U/L, Alk P 284 U/L], with esophageal varices and biopsy showing nodular regenerative hyperplasia; slow improvement upon stopping).
- Blogowski W, Marlicz W, Smereczynski A, Lawniczak M, Lewosiuk A, Starzynska T. Nodular regenerative liver hyperplasia as a complication of azathioprine-containing immunosuppressive treatment for Crohn's disease. Immunopharmacol Immunotoxicol 2011; 33: 398-402. [PubMed: 20726808](40 year old woman with Crohn disease developed nodular regenerative hyperplasia [splenomegaly and varices] 3 years after starting azathioprine).
- van Asseldonk DP, Jharap B, Kuik DJ, de Boer NK, Westerveld BD, Russel MG, Kubben FJ, et al. Prolonged thioguanine therapy is well tolerated and safe in the treatment of ulcerative colitis. Dig Liver Dis 2011; 43: 110-5. [PubMed: 20739231](Among 46 patients switched from other thiopurines to thioguanine and treated for an average of 2 years, none developed hepatotoxicity and liver biopsies done in 12 showed no evidence of nodular regeneration).
- Seksik P, Mary JY, Beaugerie L, Lémann M, Colombel JF, Vernier-Massouille G, Cosnes J. Incidence of nodular regenerative hyperplasia in inflammatory bowel disease patients treated with azathioprine. Inflamm Bowel Dis 2011; 17: 565-72. [PubMed: 20848502](Among 1888 patients with inflammatory bowel disease treated with azathioprine for median of 2.5 years, 15 developed nodular regenerative hyperplasia, 0.6% at 5 and 1.3% at 10 years; predictive factors were male gender and history of small bowel resection).
- Adam de Beaumais T, Fakhoury M, Medard Y, Azougagh S, Zhang D, Yakouben K, Jacqz-Aigrain E. Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol 2011;71: 575-84. [PMC free article: PMC3080646] [PubMed: 21395650](66 children with acute leukemia treated with mercaptopurine were tested for thiopurine methyltransferase [TPMT] and inosine pyrophosphatase (ITPA) polymorphisms and for serum levels of mercaptopurine and metabolites; those with higher levels of methyl metabolites [which varied by ITPA genotype] were more likely to have hepatotoxicity, present in 84% of participants).
- Calabrese E, Hanauer SB. Assessment of non-cirrhotic portal hypertension associated with thiopurine therapy in inflammatory bowel disease. J Crohns Colitis 2011; 5: 48-53. [PubMed: 21272804](Review of thiopurine toxicity and its management, highlighting a case of nodular regenerative hyperplasia in a patient with Crohn's colitis treated with mercaptopurine).
- Srirajaskanthan R, Valliani D. Azathioprine induced hepatitis in patients with inflammatory bowel disease. Int J Clin Pharm 2011; 33: 724-5. [PubMed: 21892694](Two patients developed symptoms, jaundice and ALT elevations on azathioprine therapy, both having normal TPMT levels; few details given).
- Masia R, Pratt DS, Misdraji J. A histopathologic pattern of centrilobular hepatocyte injury suggests 6-mercaptopurine-induced hepatotoxicity in patients with inflammatory bowel disease. Arch Pathol Lab Med 2012; 136: 618-22. [PubMed: 22646267](Review of liver biopsies from 3 patients with mercaptopurine induced liver injury showed a similar pattern centrilobular injury, minimal inflammation, ceroid-laden macrophages and steatosis, without nodular regeneration or sinusoidal dilatation).
- Tack GJ, van Asseldonk DP, van Wanrooij RL, van Bodegraven AA, Mulder CJ. Tioguanine in the treatment of refractory coeliac disease--a single centre experience. Aliment Pharmacol Ther 2012; 36: 274-81. [PubMed: 22646133](Among 12 patients with refractory celiac disease treated with thioguanine for 3 weeks to 8 years, one developed liver test abnormalities at 9 months, which resolved only when thioguanine was stopped 8 months later).
- Costantino G, Furfaro F, Belvedere A, Alibrandi A, Fries W. Thiopurine treatment in inflammatory bowel disease: response predictors, safety, and withdrawal in follow-up. J Crohns Colitis 2012; 6: 588-96. [PubMed: 22398045](Retrospective analysis of 266 patients with IBD treated with azathioprine or mercaptopurine of whom 32.5% had at least one adverse event, liver toxicity occurring in 23 patients [9%], 5 within 0-4 weeks, 12 after 2-6 months, and 6 after 6 months; specific details not given).
- Smith MA, Blaker P, Marinaki AM, Anderson SH, Irving PM, Sanderson JD. Optimising outcome on thiopurines in inflammatory bowel disease by co-prescription of allopurinol. J Crohns Colitis 2012; 6: 905-12. [PubMed: 22386736](Analysis of 110 patients with IBD treated with combination of a thiopurine and allopurinol, including 25 switched from thiopurine monotherapy because of hepatotoxicity, 20 of whom tolerated therapy with normalization of liver test abnormalities).
- Hoentjen F, Hanauer SB, de Boer NK, Rubin DT. Two brothers with skewed thiopurine metabolism in ulcerative colitis treated successfully with allopurinol and mercaptopurine dose reduction. Dig Dis Sci 2012; 57: 250-3. [PMC free article: PMC3253335] [PubMed: 22147254](Two brothers with ulcerative colitis had high 6-MMP levels and ALT elevations [114 and 141 U/L] during mercaptopurine therapy, which returned to undetectable [6-MMP] or normal [ALT] when switched to low dose mercaptopurine [25 mg/day] and allopurinol).
- Hoentjen F, Seinen ML, Hanauer SB, de Boer NK, Rubin DT, Bouma G, Harrell LE, et al. Safety and effectiveness of long-term allopurinol-thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 363-9. [PubMed: 22605661](Among 77 patients with IBD and thiopurine resistance or intolerance who were treated with allopurinol and low dose thiopurines, 6-TGN levels increased while 6-MMP levels decreased and serum ALT levels improved in 34 of 42 patients [81%], with elevations on standard dose thiopurines without allopurinol).
- Musumba CO. Review article: the association between nodular regenerative hyperplasia, inflammatory bowel disease and thiopurine therapy. Aliment Pharmacol Ther 2013; 38: 1025-37. [PubMed: 24099468](Systematic review of the literature on association of nodular regenerative hyperplasia and thiopurine use in inflammatory bowel disease, suggests the rate is as high as 62% with higher doses [>40 mg daily], but is rare at lower doses [<20 mg daily]).
- Selvaraj SA, Chairez E, Wilson LM, Lazarev M, Bass EB, Hutfless S. Use of case reports and the Adverse Event Reporting System in systematic reviews: overcoming barriers to assess the link between Crohn's disease medications and hepatosplenic T-cell lymphoma. Syst Rev 2013; 2: 53. [PMC free article: PMC3710465] [PubMed: 23826928](Review of the literature and spontaneous reports made to the FDA of HSTCL and medication use, identified 37 cases, ages 12 to 79 [mean 30] years; 86% male; disease duration 4 to 35 [mean 10] years; 96% having received thiopurines, 76% biologics; survival poor [8%]).
- Bašić Kinda S, Duraković N, Dotlić S, Serventi Seiwerth R, Davidović Mrsić S, Dubravčić K, Aurer I. Hepatosplenic αβ T-cell lymphoma arising after long-term azathioprine therapy successfully treated with allogeneic bone marrow transplant. Leuk Lymphoma 2013; 54: 1334-5. [PubMed: 23083012](Report of a patient with Crohn disease who developed HSTCL after 7 years of azathioprine therapy, few details provided).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to thioguanine, azahioprine or mercaptopurine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 10 cases [1.3%] were attributed to azathioprine, [1.2%] to mercaptopurine but none to thioguanine).
- Botros Y, Mathews M, Patel H, Shah N, Baddoura W, de la Torre A. Recurrent hepatocellular carcinoma in patient with Crohn's disease: incidental or expected outcome of azathioprine? Case Rep Gastrointest Med 2015; 2015: 939136. [PMC free article: PMC4691603] [PubMed: 26788381](62 year old man with Crohn disease and azathioprine therapy for 21 years, developed HCC with recurrence after resection; no mention of cirrhosis).
- Brinkert F, Arrenberg P, Krech T, Grabhorn E, Lohse A, Schramm C. Two cases of hepatosplenic T-cell lymphoma in adolescents treated for autoimmune hepatitis. Pediatrics 2016; 138. pii: e20154245. [PubMed: 27516526](Two patients who developed HSTCL during azathioprine therapy of autoimmune hepatitis: 21 year old man treated for 5 years and an 18 year old female treated for 4 years, both presenting with hepatosplenomegaly, fever and pancytopenia).
- Suárez Ferrer C, Llop Herrera E, Calvo Moya M, Vera Mendoza MI, González Partida I, González Lama Y, Matallana Royo V, et al. Idiopathic portal hypertension regarding thiopurine treatment in patients with inflammatory bowel disease. Rev Esp Enferm Dig 2016; 108: 79-83. [PubMed: 26838489](Among 1419 patients with inflammatory bowel disease followed at a referral center in Spain, 927 were treated with thiopurines, of whom 63 [7%] developed liver injury, mostly hepatotoxicity that resolved with dose modification or discontinuation, but 4 had portal hypertension and probable nodular regenerative hyperplasia [ages 42-72 years, treated for 2.5-10 years, bilirubin 0.4-2.2 mg/dL, ALT 21-65 U/L, platelet counts 60,000-100,000]).
- van de Meeberg MM, Derikx LA, Sinnige HA, Nooijen P, Schipper DL, Nissen LH. Hepatosplenic T-cell lymphoma in a 47-year-old Crohn's disease patient on thiopurine monotherapy. World J Gastroenterol 2016; 22: 10465-70. [PMC free article: PMC5175260] [PubMed: 28058028](47 year old man with Crohn disease on azathioprine therapy developed HSTCL, few details provided).
- Heron V, Fortinsky KJ, Spiegle G, Hilzenrat N, Szilagyi A. Resected hepatocellular carcinoma in a patient with Crohn's disease on azathioprine. Case Rep Gastroenterol 2016; 10: 50-6. [PMC free article: PMC4929375] [PubMed: 27403102](61 year old woman with 30 year history of Crohn disease and 10 years of azathioprine therapy presented with liver mass with HCC on biopsy without cirrhosis, but no malignancy at time of resection several months later).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Azathioprine.[LiverTox: Clinical and Researc...]Review Azathioprine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Sustained effectiveness, safety and therapeutic drug monitoring of tioguanine in a cohort of 274 IBD patients intolerant for conventional therapies.[Aliment Pharmacol Ther. 2019]Sustained effectiveness, safety and therapeutic drug monitoring of tioguanine in a cohort of 274 IBD patients intolerant for conventional therapies.Simsek M, Deben DS, Horjus CS, Bénard MV, Lissenberg-Witte BI, Buiter HJC, van Luin M, Seinen ML, Mulder CJJ, Wong DR, et al. Aliment Pharmacol Ther. 2019 Jul; 50(1):54-65. Epub 2019 May 16.
- Toxicity of 6-thioguanine: no hepatotoxicity in a series of IBD patients treated with long-term, low dose 6-thioguanine. Some evidence for dose or metabolite level dependent effects?[Dig Liver Dis. 2007]Toxicity of 6-thioguanine: no hepatotoxicity in a series of IBD patients treated with long-term, low dose 6-thioguanine. Some evidence for dose or metabolite level dependent effects?Gilissen LP, Derijks LJ, Driessen A, Bos LP, Hooymans PM, Stockbrügger RW, Engels LG. Dig Liver Dis. 2007 Feb; 39(2):156-9. Epub 2006 Dec 26.
- Thioguanine used in maintenance therapy of chronic myeloid leukaemia causes non-cirrhotic portal hypertension. Results from MRC CML. II. Trial comparing busulphan with busulphan and thioguanine.[Br J Haematol. 1991]Thioguanine used in maintenance therapy of chronic myeloid leukaemia causes non-cirrhotic portal hypertension. Results from MRC CML. II. Trial comparing busulphan with busulphan and thioguanine.Shepherd PC, Fooks J, Gray R, Allan NC. Br J Haematol. 1991 Oct; 79(2):185-92.
- Review Effectiveness and safety of thioguanine as a maintenance therapy of inflammatory bowel disease: Systematic review, meta-analysis and meta-regression.[Clin Res Hepatol Gastroenterol...]Review Effectiveness and safety of thioguanine as a maintenance therapy of inflammatory bowel disease: Systematic review, meta-analysis and meta-regression.Jena A, Neelam PB, Telaprolu H, Mangipudi UK, Dutta U, Sebastian S, Sharma V. Clin Res Hepatol Gastroenterol. 2023 Aug; 47(7):102155. Epub 2023 Jun 8.
- Thioguanine - LiverToxThioguanine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...