NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nelarabine is a purine analogue and antineoplastic agent used in the therapy of T cell lymphoblastic leukemia or lymphoma. Nelarabine is associated with a low rate of transient serum enzyme elevations during therapy and has been linked to rare instances of clinically apparent acute liver injury with jaundice.
Background
Nelarabine (ne lar' a been) is a purine analogue that is used in the treatment of T cell leukemia or lymphoma. Nelarabine is arabinosyl derivative of deoxyguanosine (2-amino-9-beta-D-arabinofuranosyl-6-methoxy-9-H-guanine) that, after removal of the methoxy group by adenosine deaminase in serum, is taken up and converted intracellularly to the active triphosphate, which is believed to compete with guanine triphosphate for use by DNA polymerase leading to inhibition of DNA synthesis. It has selective activity against T lymphocytes and was found to have activity against acute T cell malignancies. Nelarabine was approved for use as an antineoplastic agent in the United States in 2005. Current indications are therapy of acute T cell lymphoblastic leukemia and T cell lymphoblastic lymphoma after failure of prior therapies. Nelarabine is available as a solution for injection under the trade name Arranon. The typical adult dose is 1500 mg/m2 intravenously on days 1, 3 and 5 of 21-day cycles. Common side effects include bone marrow suppression, nausea, vomiting, anorexia, diarrhea, headache, fatigue, mucositis and skin rash. The major dose limiting side effects of nelarabine are neurologic, including somnolence, headache, dizziness, ataxia, delirium, seizures, neuropathy and Guillain Barre syndrome. The neurologic toxicities can be severe and some are not reversible on stopping nelarabine as is stressed by the boxed warning in the product label.
Hepatotoxicity
In clinical trials, serum enzymes elevations occurred in a small proportion of patients treated with nelarabine when given as sole therapy for refractory or relapsed acute leukemia. These elevations are generally mild-to-moderate, transient and asymptomatic. Elevations of aminotransferase levels above 5 times the upper limit of normal are reported in 4% of patients with leukemia receiving nelarabine. The elevations rarely require dose adjustment or delay in therapy. Cases of clinically apparent liver injury due to nelarabine have been reported to occur, but few details are available. A single case report of clinically apparent liver injury attributed to nelarabine has been published with rapid onset of jaundice during a second course of nelarabine, a hepatocellular pattern of enzyme elevations, no immunoallergic or autoimmune features and a rapid improvement upon stopping.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury).
Mechanism of Injury
Hepatotoxicity from nelarabine is likely due to direct toxicity as is typical for other purine analogues.
Outcome and Management
The severity of the liver injury linked to nelarabine therapy is generally self-limited and mild and resolves with stopping therapy. There is little evidence of cross sensitivity to liver injury among the various antineoplastic or antiviral purine analogues.
Drug Class: Antineoplastic Agents, Antimetabolites
Other Drugs in the Subclass, Purine Analogues: Azathioprine, Cladribine, Clofarabine, Fludarabine, Mercaptopurine, Pentostatin, Thioguanine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nelarabine – Generic, Arranon®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
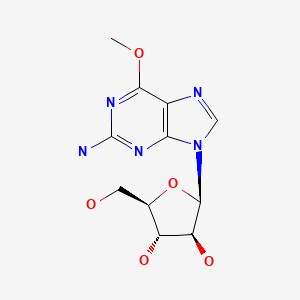
| Nelarabine | 121032-29-9 | C11-H15-N5-O5 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 01 May 2020
Abbreviations: ALL, acute lymphocyte leukemia; HCT, hematopoietic cell transplantation.
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999 discusses the purine analogues pentostatin, cladribine and fludarabine but not clofarabine or nelarabine).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 541-68.(Review of hepatotoxicity of hepatotoxicity of anticancer agents does not discuss nelarabine).
- Chabner BA, Bertino J, Cleary J, Ortiz T, Lane A, Supko JG, Ryan DP. Purine analogs. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1701-5.(Textbook of pharmacology and therapeutics).
- Kisor DF. Nelarabine: a nucleoside analog with efficacy in T-cell and other leukemias. Ann Pharmacother. 2005;39:1056–63. [PubMed: 15870141](Analysis of structure, mechanism of action, pharmacokinetics, efficacy and safety of nelarabine in T cell malignancies mentions that the dose limiting toxicity is neurological; no discussion of hepatotoxicity).
- Gandhi V, Plunkett W. Clofarabine and nelarabine: two new purine nucleoside analogs. Curr Opin Oncol. 2006;18:584–90. [PubMed: 16988579](Review of clofarabine and nelarabine, both of which had been recently approved for use in ALL and T cell leukemia or lymphoma, dose limiting toxicities being hepatic for clofarabine, neurologic for nelarabine).
- Czuczman MS, Porcu P, Johnson J, Niedzwiecki D, Kelly M, Hsi ED, Cook JR, et al. Cancer and Leukemia Group B. Results of a phase II study of 506U78 in cutaneous T-cell lymphoma and peripheral T-cell lymphoma: CALGB 59901. Leuk Lymphoma. 2007;48:97–103. [PubMed: 17325852](Among 18 adults with T cell lymphomas treated with nelarabine, none had ALT or AST elevations during therapy, but neurologic toxicity was common [33%] and dose limiting).
- Buie LW, Epstein SS, Lindley CM. Nelarabine: a novel purine antimetabolite antineoplastic agent. Clin Ther. 2007;29:1887–99. [PubMed: 18035189](Review of the structure, mechanism of action, pharmacokinetics, clinical efficacy and safety of nelarabine encountered in preapproval trials; discusses the neurologic and hematologic, but not hepatic toxicities of nelarabine).
- Cohen MH, Johnson JR, Justice R, Pazdur R. FDA drug approval summary: nelarabine (Arranon) for the treatment of T-cell lymphoblastic leukemia/lymphoma. Oncologist. 2008;13:709–14. [PubMed: 18586926](Summary of the evidence for efficacy and safety of nelarabine in T cell leukemia and lymphoma which led to its approval in refractory or relapsed cases; the major dose limiting toxicity was neurologic, elevations of ALT levels above 5 times the ULN occurred in 4% of patients; no mention of clinically apparent liver injury).
- Iino M. Rinsho Ketsueki. 2009;50:49–51. [Severe liver injury following nelarabine chemotherapy for T-cell lymphoblastic lymphoma] Japanese. [PubMed: 19225230](41 year old man with T cell lymphoma after HCT developed liver test abnormalities at the end of a second course of nelarabine [peak bilirubin 17.7 mg/dL, ALT 2149 U/L, Alk P 704 U/L], resolving within 1 month of stopping with prednisolone and ursodiol treatment).
- Commander LA, Seif AE, Insogna IG, Rheingold SR. Salvage therapy with nelarabine, etoposide, and cyclophosphamide in relapsed/refractory paediatric T-cell lymphoblastic leukaemia and lymphoma. Br J Haematol. 2010;150:345–51. [PubMed: 20528871](Among 7 children with T cell leukemia treated with nelarabine in combination with etoposide and cyclophosphamide, 6 developed neurological toxicities, mostly pain, headache, somnolence, dizziness, neuropathy, tremor and ataxia; no mention of ALT elevations or hepatotoxicity).
- Dunsmore KP, Devidas M, Linda SB, Borowitz MJ, Winick N, Hunger SP, Carroll WL, et al. Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:2753–9. [PMC free article: PMC3402886] [PubMed: 22734022](Among 92 children with T cell leukemia, 72 received nelarabine in addition of standard therapy; ALT and AST elevations occurred in 44% on nelarabine and in 56% who were not; neurologic side effects were more common with nelarabine, 11 developing neuropathy, 4 seizures and 1 a Guillain Barre-like syndrome).
- Winter SS, Dunsmore KP, Devidas M, Eisenberg N, Asselin BL, Wood BL, Leonard Rn MS, et al. Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: Children's Oncology Group Study AALL0434. Pediatr Blood Cancer. 2015;62:1176–83. [PMC free article: PMC4433576] [PubMed: 25755211](Among 94 patients with acute T-cell leukemia or lymphoma treated with standard chemotherapy [usually methotrexate and pegaspargase] with or without nelarabine, rates of neuropathy were similar with or without nelarabine; no mention of ALT elevations or hepatotoxicity).
- Zwaan CM, Kowalczyk J, Schmitt C, Bielorai B, Russo MW, Woessner M, Ranganathan S, et al. Safety and efficacy of nelarabine in children and young adults with relapsed or refractory T-lineage acute lymphoblastic leukaemia or T-lineage lymphoblastic lymphoma: results of a phase 4 study. Br J Haematol. 2017;179:284–93. [PubMed: 28771663](Among 28 children or young adults with acute T cell leukemia or lymphoma treated with 1-5 cycles of nelarabine, median survival was 6.5 months and adverse events were common, with 5 cases of neurologic adverse events but ALT elevations above 5 times the ULN in only one patient).
- Kadia TM, Gandhi V. Nelarabine in the treatment of pediatric and adult patients with T-cell acute lymphoblastic leukemia and lymphoma. Expert Rev Hematol. 2017;10:1–8. [PMC free article: PMC5578611] [PubMed: 27869523](Review of the published literature on the efficacy and safety of nelarabine as therapy of acute T-cell leukemia and lymphoma, mentions the hematologic and neurologic toxicities which are frequent and are dose related; no mention of ALT elevations or hepatotoxicity).
- Abaza Y, M, Kantarjian H, Faderl S, Jabbour E, Jain N, Thomas D, Kadia T, et al. Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma. Am J Hematol. 2018;93:91–9. [PubMed: 29047158](Among 67 patients with acute T-cell leukemia or lymphoma treated with hyper-CVAD and nelarabine, the response rate was 96% and 3 year survival 66%, while adverse events occurred in all patients and were grade 3 or above in 91% with ALT elevations arising in 91% which were above 5 times ULN in 27%; no mention of clinically apparent hepatic adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Nelarabine, etoposide, and cyclophosphamide in relapsed pediatric T-acute lymphoblastic leukemia and T-lymphoblastic lymphoma (study T2008-002 NECTAR).[Pediatr Blood Cancer. 2022]Nelarabine, etoposide, and cyclophosphamide in relapsed pediatric T-acute lymphoblastic leukemia and T-lymphoblastic lymphoma (study T2008-002 NECTAR).Whitlock JA, Malvar J, Dalla-Pozza L, Goldberg JM, Silverman LB, Ziegler DS, Attarbaschi A, Brown PA, Gardner RA, Gaynon PS, et al. Pediatr Blood Cancer. 2022 Nov; 69(11):e29901. Epub 2022 Aug 21.
- Review Nelarabine in the treatment of pediatric and adult patients with T-cell acute lymphoblastic leukemia and lymphoma.[Expert Rev Hematol. 2017]Review Nelarabine in the treatment of pediatric and adult patients with T-cell acute lymphoblastic leukemia and lymphoma.Kadia TM, Gandhi V. Expert Rev Hematol. 2017 Jan; 10(1):1-8. Epub 2016 Dec 8.
- Review Nelarabine: a novel purine antimetabolite antineoplastic agent.[Clin Ther. 2007]Review Nelarabine: a novel purine antimetabolite antineoplastic agent.Buie LW, Epstein SS, Lindley CM. Clin Ther. 2007 Sep; 29(9):1887-99.
- A phase 1 study to evaluate the safety, pharmacology, and feasibility of continuous infusion nelarabine in patients with relapsed and/or refractory lymphoid malignancies.[Cancer. 2023]A phase 1 study to evaluate the safety, pharmacology, and feasibility of continuous infusion nelarabine in patients with relapsed and/or refractory lymphoid malignancies.Boddu PC, Senapati J, Ravandi-Kashani F, Jabbour EJ, Jain N, Ayres M, Chen Y, Keating MJ, Kantarjian HM, Gandhi V, et al. Cancer. 2023 Feb 15; 129(4):580-589. Epub 2022 Nov 29.
- Review Nelarabine-associated reversible Guillain-Barré-like syndrome or myelopathy in an adult patient with primary refractory T-lymphoblastic lymphoma.[Curr Probl Cancer. 2017]Review Nelarabine-associated reversible Guillain-Barré-like syndrome or myelopathy in an adult patient with primary refractory T-lymphoblastic lymphoma.Lalayanni C, Baldoumi E, Papayiannopoulos S, Tziola K, Saloum R, Anagnostopoulos A. Curr Probl Cancer. 2017 Mar-Apr; 41(2):138-143. Epub 2016 Nov 17.
- Nelarabine - LiverToxNelarabine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...