NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Bosutinib is a dual kinase inhibitor of both the BCR-ABL and Src tyrosine kinases and is used in the therapy of Philadelphia chromosome-positive chronic myelogenous leukemia. Bosutinib therapy is associated with transient elevations in serum aminotransferase and bilirubin levels and rare instances of clinically apparent acute liver injury.
Background
Bosutinib (boe sue’ ti nib) is an orally available, small molecule inhibitor of the BCR-ABL tyrosine kinase receptor, which is the product of a fusion gene resulting from the reciprocal translocations between chromosomes 9 and 22 that characterize the Philadelphia chromosome of chronic myelogenous leukemia (CML). The abnormal tyrosine kinase receptor is constitutively expressed and causes unregulated cell growth and proliferation in myeloid cells. Inhibition of the receptor can lead to dramatic reversal of progression of leukemia, although sometimes limited by the development of tumor resistance caused by mutations in the kinase. Bosutinib was the fourth BCR-ABL tyrosine kinase receptor inhibitor approved for use in cancer chemotherapy (after imatinib, dasatinib and nilotinib) and, like many of the BCR-ABL tyrosine kinase inhibitors, also has some activity against the abnormal tyrosine kinase (c-Kit) that is found in gastrointestinal stromal tumors (GIST). Bosutinib also has activity against the receptors for platelet derived growth factor (PDGF) and vascular endothelial growth factor (VEGF). Bosutinib received approval for use in the United States in 2012. Current indications are for adult patients with Philadelphia chromosome-positive CML in the chronic, accelerated or blast stages and with resistance or intolerance to previous therapy. Bosutinib is available in tablets of 100 and 500 mg under the brand name Bosulif. The typical dose is 500 mg orally once daily, with dose adjustment based upon tolerance and efficacy. Side effects include fatigue, nausea, diarrhea, abdominal pain, thrombocytopenia, anemia, fever and rash. Uncommon, but potentially severe side effects include hypersensitivity reactions, infections and bone marrow suppression.
Hepatotoxicity
In large clinical trials of bosutinib, elevations in serum aminotransferase levels were common, occurring in up to 58% of bosutinib treated patients. Values greater than 5 times the upper limit of normal (ULN) occurred in 4% to 19% of bosutinib recipients (and 3% of imatinib treated subjects). These abnormalities were usually asymptomatic, but led to discontinuation of therapy in up to 2% of treated patients. In addition, there have been isolated reports of clinically apparent liver injury attributed to bosutinib therapy, although the frequency of this outcome and the clinical features of the injury have not been well defined. The time to onset has generally been within 3 months and the pattern of serum enzyme elevations was usually hepatocellular.
Certainly other tyrosine kinase receptor inhibitors used in the therapy of CML such as imatinib, nilotinib and ponatinib have been associated with cases of acute liver injury with jaundice. With these agents, the liver injury typically arises after several months of therapy and the pattern of serum enzyme elevations is typically hepatocellular. Immunoallergic features (rash, fever and eosinophilia) and autoantibody formation are usually not present.
Reactivation of hepatitis B has been reported with imatinib and nilotinib therapy, but not with bosutinib. Reactivation typically occurs in an HBsAg positive person treated with the tyrosine kinase inhibitor for 3 to 6 months, presenting with jaundice, marked serum aminotransferase elevations and an increase in HBV DNA levels. Reactivation of hepatitis B can be severe and fatal instances have been reported after imatinib and nilotinib therapy. Screening of patients for HBsAg and anti-HBc is sometimes recommended before starting cancer chemotherapy and those with HBsAg offered prophylaxis with oral antiviral agents, such as lamivudine, tenofovir or entecavir. Whether reactivation occurs with bosutinib therapy is unclear.
Likelihood score: D (possible uncommon cause of clinically apparent liver injury).
Mechanism of Injury
The cause of liver injury due to bosutinib is not known. Bosutinib is metabolized in the liver largely through the CYP 3A4 pathway and liver injury may be related to production of a toxic intermediate. Bosutinib is susceptible to drug-drug interactions with medications that are metabolized by CYP 3A4 or induce or inhibit CYP 3A4 activity.
Outcome and Management
The product label for bosutinib recommends monitoring liver tests monthly for the first 3 months of treatment and as clinically indicated therafter. Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) and any elevation associated with jaundice or symptoms should lead to dose reduction or temporary cessation. In some situations, therapy can be restarted, particularly with concurrent prednisone (10 to 20 mg daily). In patients with clinically apparent liver injury and jaundice, restarting therapy should be done with caution. There does not appear to be cross reactivity in risk for hepatic injury between bosutinib and other tyrosine kinase inhibitors and, in some situations, switching to another BCR-ABL inhibitor may be appropriate. In using this medication, other potentially hepatotoxic agents should be avoided.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
Other Drugs in the Subclass, Chronic Myeloid Leukemia Agents: Dasatinib, Imatinib, Nilotinib, Omacetaxine, Ponatinib
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Bosutinib – Bosulif®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Bosutinib | 380843-75-4 | C26-H29-Cl2-N5-O3 |
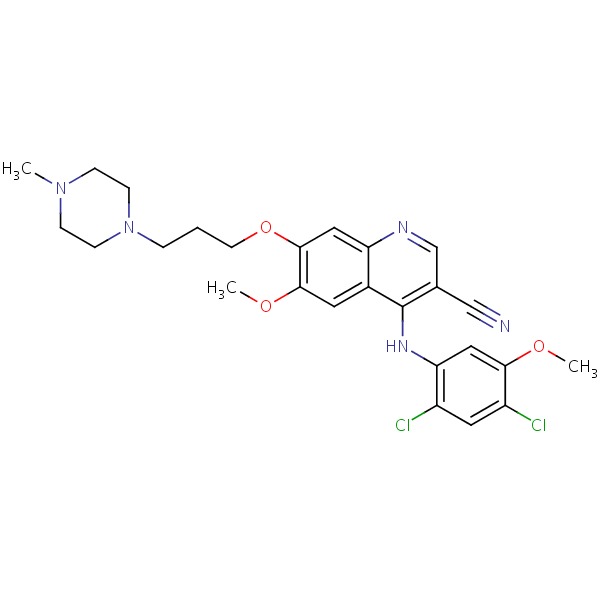 |
ANNOTATED BIBLIOGRAPHY
References updated: 30 September 2017
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of tyrosine kinase receptor inhibitors such as imatinib and bosutinib).
- DeLeve LD. Erlotinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents published; bosutinib is not discussed).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagic H, Sequist L, Wilson W, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-54.(Textbook of pharmacology and therapeutics).
- Cortes JE, Kantarjian HM, Brümmendorf TH, Kim DW, Turkina AG, Shen ZX, Pasquini R, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 2011; 118: 4567-76. [PMC free article: PMC4916618] [PubMed: 21865346](Among 288 patients with CML resistant or intolerant to imatinib treated with bosutinib for an average of 2 years, 10% had ALT elevations above 5 times ULN, typically arising during the first month of therapy, but only 2% required drug discontinuation because of the elevations).
- Campone M, Bondarenko I, Brincat S, Hotko Y, Munster PN, Chmielowska E, Fumoleau P, et al. Phase II study of single-agent bosutinib, a Src/Abl tyrosine kinase inhibitor, in patients with locally advanced or metastatic breast cancer pretreated with chemotherapy. Ann Oncol 2012; 23: 610-7. [PubMed: 21700731](Among 73 patients with advanced breast cancer treated with bosutinib for up to 12 months, side effects were common and ALT elevations occurred in more than half of patients and were ≥5 times ULN in 16%).
- Stansfield L, Hughes TE, Walsh-Chocolaad TL. Bosutinib: a second-generation tyrosine kinase inhibitor for chronic myelogenous leukemia. Ann Pharmacother 2013; 47: 1703-11. [PubMed: 24396109](Review of pharmacology, efficacy and safety of bosutinib as therapy of CML, mentions that adverse events included diarrhea [68%], vomiting [32%] and ALT elevations [22%]).
- Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf 2013; 36: 491-503. [PubMed: 23620168](Review of the hepatotoxicity of 18 tyrosine kinase inhibitors approved for use in cancer in the US as of 2013; aminotransferase elevations occurred in 20% of patients in registration trials of bosutinib [ ≥5 times ULN in 4-9%], and at least one case of clinically apparent hepatitis was reported).
- Gambacorti-Passerini C, Cortes JE, Lipton JH, Dmoszynska A, Wong RS, Rossiev V, Pavlov D, et al. Safety of bosutinib versus imatinib in the phase 3 BELA trial in newly diagnosed chronic phase chronic myeloid leukemia. Am J Hematol 2014 Jun 18. [Epub ahead of print] [PMC free article: PMC4305212] [PubMed: 24944159](Comparison of imatinib vs bosutinib in 502 adults with CML found higher rates of adverse effects with bosutinib, including diarrhea [70% vs 26%], vomiting [33% vs 16%], ALT elevations [33% vs 9%], ALT elevations ≥5 times ULN [19% vs 3%] and fever [19% vs 12%]).
- Gambacorti-Passerini C, Brümmendorf TH, Kim DW, Turkina AG, Masszi T, Assouline S, Durrant S, et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: Minimum 24-month follow-up. Am J Hematol 2014; 89: 732-42. [PMC free article: PMC4173127] [PubMed: 24711212](Data from 2 year follow up of treatment of 288 patients with chronic phase CML resistant or intolerant to imatinib, found complete responses in 85% of patients; ALT elevations occurred in 58%, were ≥5 times ULN in 10% and led to discontinuation in 2% of patients).
- Hanaizi Z, Unkrig C, Enzmann H, Camarero J, Sancho-Lopez A, Salmonson T, Gisselbrecht C, et al. The European medicines agency review of bosutinib for the treatment of adult patients with chronic myelogenous leukemia: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist 2014; 19: 421-5. [PMC free article: PMC3983815] [PubMed: 24668331](The basis of approval of bosutinib as therapy of CML previously treated with a tyrosine kinase inhibitor; side effects were common and included AST elevations in 18% of patients with rise above 5 times ULN in 44 [5%]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 [5%] were attributed to antineoplastic agents including 5 due to imatinib, but none to bosutinib, dasatinib or nilotinib).
- Brümmendorf TH, Cortes JE, de Souza CA, Guilhot F, Duvillié L, Pavlov D, Gogat K, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol. 2015; 168: 69-81. [PMC free article: PMC4274978] [PubMed: 25196702](Further follow up on 502 patients with CML treated in a phase 3 clinical trial of bosutinib vs imatinib [Gambacorti-Passerini 2014], found similar 3-4 year survival rates, but with higher rates of ALT elevations [23% vs 4%] and discontinuations for ALT abnormalities [4% vs <1%] with bosutinib).
- Tesar V, Ciechanowski K, Pei Y, Barash I, Shannon M, Li R, Williams JH, et al. Bosutinib versus placebo for autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2017; 28 (11): 3404-13. [PMC free article: PMC5661280] [PubMed: 28838955](Among 172 patients with autosomal dominant polycystic kidney disease treated with bosutinib [200 or 400 mg] or placebo daily for up to 2 years, discontinuations were frequent and ALT elevations occurred in 42% on bosutinib vs 7% on placebo which were above 5 times ULN in 9% vs 2%; one subject on bosutinib developed acute hepatitis with jaundice).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Bosutinib in the management of chronic myelogenous leukemia.[Biologics. 2013]Bosutinib in the management of chronic myelogenous leukemia.Amsberg GK, Schafhausen P. Biologics. 2013; 7:115-22. Epub 2013 May 6.
- Review Bosutinib: a review of its use in patients with Philadelphia chromosome-positive chronic myelogenous leukemia.[BioDrugs. 2014]Review Bosutinib: a review of its use in patients with Philadelphia chromosome-positive chronic myelogenous leukemia.Syed YY, McCormack PL, Plosker GL. BioDrugs. 2014 Feb; 28(1):107-20.
- Review Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia.[Clin Ther. 2007]Review Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia.Steinberg M. Clin Ther. 2007 Nov; 29(11):2289-308.
- Review Bosutinib.[Recent Results Cancer Res. 2010]Review Bosutinib.Keller G, Schafhausen P, Brümmendorf TH. Recent Results Cancer Res. 2010; 184:119-27.
- Bosutinib for the Treatment of Philadelphia Chromosome-Positive Leukemias.[Expert Opin Orphan Drugs. 2015]Bosutinib for the Treatment of Philadelphia Chromosome-Positive Leukemias.Varallo-Rodriguez C, Freyer CW Jr, Ontiveros EP, Griffiths EA, Wang ES, Wetzler M. Expert Opin Orphan Drugs. 2015; 3(5):599-608. Epub 2015 Apr 16.
- Bosutinib - LiverToxBosutinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...