NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Imatinib is specific tyrosine kinase receptor inhibitor that is used in the therapy of Philadelphia chromosome-positive chronic myelogenous leukemia and gastrointestinal stromal tumors, both of which are marked by an abnormal, constitutively expressed tyrosine kinase that causes unregulated cell growth. Imatinib therapy is associated with transient elevations in serum aminotransferase levels and rare instances of clinically apparent acute liver injury which can be severe and sometimes fatal.
Background
Imatinib (im a' ti nib) is a specific inhibitor of the unique bcr-abl tyrosine kinase receptor, which is the product of a fusion gene resulting from the translocation between chromosomes 9 and 22 that characterizes the Philadelphia chromosome (Ph) of chronic myelogenous leukemia (CML). The abnormal tyrosine kinase receptor is constitutively expressed and causes abnormal cell growth and proliferation. Introduction of imatinib into clinical medicine was an important advance in the therapy of cancer, the first antineoplastic agent specially directed at a molecular abnormality that occurs in cancer cells. Inhibition of the enzyme can lead to dramatic reversal of progression of Ph+ leukemia and is highly effective, although limited by the development of tumor resistance caused by mutations in the kinase. A similar abnormal tyrosine kinase (cKit) is also found in gastrointestinal stromal tumors (GIST). Imatinib received accelerated approval for use in the United States in May 2001 and subsequent full approval in December 2005. Imatinib is available in tablets of 100 and 400 mg generically and under the brand name Gleevec. Current indications are for Philadelphia chromosome positive (Ph+) CML and Ph+ acute lymphoblastic leukemia, for unresectable or metastatic GIST with positive Kit (CD117) and for other malignant syndromes with other kinase mutations sensitive to imatinib including myelodysplastic disorders and systemic mastocytosis. The typical dose is 400 to 600 mg once daily. Side effects include nausea, edema, muscle cramps, arthralgias, fatigue, fever, headache, abdominal discomfort, diarrhea, anemia, cough, rash and pruritus. Rare, but potentially severe adverse events include severe myelosuppression, congestive heart failure, cardiogenic shock, hemorrhage, severe rash, embryo-fetal toxicity, tumor lysis syndrome, renal toxicity and growth retardation.
Hepatotoxicity
Imatinib therapy is associated with three forms of acute liver injury: transient and usually asymptomatic elevations in serum enzymes during treatment, clinically apparent acute hepatitis, and reactivation of an underlying chronic hepatitis B.
Elevations in serum aminotransferase levels are common during imatinib therapy, but ALT levels above 5 times the upper limit of the normal range occur in only 2% to 4% of patients treated for 6 months or more. In addition, mild elevations in serum bilirubin can occur. These abnormalities are usually mild, asymptomatic, and resolve despite continuing therapy. Nevertheless, dose adjustment or temporary discontinuation and restarting at a lower dose may be needed and is recommended if levels are markedly elevated (ALT or AST persistently >5 times ULN or bilirubin >3 times ULN).
In addition, imatinib has been linked to rare instances of clinically apparent acute liver injury with jaundice. The time to onset has varied from 6 days to as long as several years after starting treatment, the usual latency being 2 to 6 months (Cases 1 and 2). The pattern of serum enzyme elevations is typically hepatocellular, although cholestatic and mixed forms of hepatitis have also been reported. The injury can be severe and instances of acute liver failure and death have been reported as well as severe hepatitis resulting in a posthepatitic cirrhosis. Immunoallergic features (rash, fever and eosinophilia) are not common, but some patients develop low levels of autoantibodies and instances of chronic hepatitis on long term imatinib have been reported. More importantly, many instances of an apparent clinical response to prednisone therapy have been described. Recurrence of injury is common with reexposure, but concurrent prednisone therapy may blunt or prevent the recurrence of liver injury and, in some instances, has allowed for continued, long term therapy despite a previous bout of clinically apparent liver injury on imatinib.
Finally, there have been several instances of reactivation of chronic hepatitis B during imatinib therapy in patients with inactive hepatitis B or the HBsAg carrier state (Case 3). The clinical presentation is generally with an acute hepatitis like syndrome with marked elevations in serum ALT and minimal changes in alkaline phosphatase levels. Typically, hepatitis B virus (HBV) DNA is present in serum in increasing levels early in the course of reactivation which rapidly falls to pretreatment levels with recovery. Patients may also test positive for IgM antibody to hepatitis B core antigen (IgM anti-HBc). Reactivation of hepatitis B due to imatinib can be severe and fatal instances have been reported.
Likelihood score: B (likely cause of clinically apparent liver injury as well as reactivation of hepatitis B).
Mechanism of Injury
The immune features and response to prednisone therapy suggest that the hepatic injury is immunologically mediated. However, there may be multiple causes of liver injury with imatinib therapy. Reactivation of hepatitis B is probably caused by immune suppression leading to increased viral replication, followed by immune recovery and acute liver injury.
Outcome and Management
Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to dose reduction or temporary cessation. In some situations, therapy can be restarted particularly with concurrent prednisone (10 mg to 20 mg daily). In patients with clinically apparent liver injury and jaundice, restarting therapy should be done with caution. Cross sensitivity to liver injury is uncommon among the tyrosine kinase inhibitors and, in many situations, switching to another tyrosine kinase inhibitor may be appropriate. Cases of acute liver failure have occurred in patients receiving imatinib. In using this medication, other potentially hepatotoxic agents should be avoided. Patients with reactivation of hepatitis B usually recover spontaneously, but therapy with an oral antiviral agent active against HBV is often used. Importantly, patients who are to receive long term therapy with imatinib should be screened for hepatitis B and, if positive, given antiviral prophylaxis against reactivation (with an oral antiviral agent with potent activity against HBV such as entecavir or tenofovir) or monitored prospectively and started on antiviral therapy if HBV DNA levels rise.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
Other Drugs in the Subclass, Chronic Myeloid Leukemia Agents: Bosutinib, Dasatinib, Nilotinib, Omacetaxine, Ponatinib
CASE REPORTS
Case 1. Severe acute hepatitis during imatinib therapy.
[Modified from: Tonyali O, Coskun U, Yildiz R, Karakan T, Demirci U, Akyurek N, Benekli M, et al. Imatinib mesylate-induced acute liver failure in a patient with gastrointestinal stromal tumors. Med Oncol 2010; 27: 768-73. PubMed Citation]
A 53 year old woman with unresectable gastrointestinal stromal tumors developed fatigue and liver test abnormalities 10 weeks after starting imatinib therapy (400 mg daily). She had no history of liver disease and was known to have had normal liver tests before starting imatinib (Table). She drank little alcohol, had no risk factors for viral hepatitis, and was taking no other medications. Laboratory tests showed marked elevations in serum aminotransferase levels (ALT 944 U/L, AST 678 U/L), with minimal increases in alkaline phosphatase (195 U/L) and normal bilirubin (1.2 mg/dL). Imatinib was stopped, but over the next week she continued to worsen. She was admitted for management of the liver injury. On admission, she was jaundiced and had a flapping tremor. Abdominal ultrasound showed mild ascites, but normal liver texture and no evidence of biliary obstruction. Tests for hepatitis A, B and C were negative as were autoimmune markers. Because of the worsening clinical syndrome, prednisolone was started (40 mg daily), whereupon she began to improve. A liver biopsy showed centrilobular necrosis, inflammation and interface hepatitis. Rapid tapering of prednisolone was followed by a transient increase in ALT levels, but eventually all liver tests returned to normal and corticosteroids were stopped. She was later treated with sunitinib (another tyrosine kinase inhibitor) without recurrence of liver injury.
Key Points
| Medication: | Imatinib (400 mg daily) |
| Pattern: | Hepatocellular (R=17.4) |
| Severity: | 4+ (jaundice, hospitalization, ascites and hepatic encephalopathy) |
| Latency: | 10 weeks |
| Recovery: | 12 weeks |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | Pre | 11 | 69 | 0.6 | Imatinib started |
| 4 weeks | Pre | 15 | 77 | 0.8 | |
| 10 weeks | 0 | 994 | 195 | 2.3 | Imatinib stopped |
| 11 weeks | 8 days | 1234 | 203 | 7.8 | Admitted |
| 9 days | 1276 | 205 | 9.8 | Prednisolone started | |
| 10 days | 1173 | 208 | 9.3 | ||
| 12 weeks | 2 weeks | 384 | 185 | 3.5 | |
| 13 weeks | 3 weeks | 199 | 3.1 | ||
| 15 weeks | 5 weeks | 246 | 140 | 1.0 | |
| 4 months | 6 weeks | 46 | 100 | 0.9 | Liver biopsy |
| 5 months | 9 weeks | 14 | 115 | 1.1 | Prednisolone stopped |
| Normal Values | <40 | <142 | <1.2 | ||
Comment
An acute viral hepatitis-like syndrome arose after 10 weeks of imatinib therapy. While autoantibodies were not detected, the hepatotoxicity of imatinib has many features suggestive of immunologically mediated injury and appears to respond to immunosuppressive therapy. The marked worsening of the clinical condition of this patient led to initiation of high dose prednisolone therapy, which was rapidly followed by clinical improvement. An attempt to lower the dose of prednisolone (from 40 to 10 mg daily) after only two weeks of treatment was followed by worsening of liver tests, and the dose was increased until serum aminotransferase levels had fallen into the normal range. Eventually, corticosteroids were stopped and there was no recurrence of liver injury.
Case 2. Acute liver failure due to imatinib.
[Modified from: Ridruejo E, Cacchione R, Villamil AG, Marciano S, Gadano AC, Mandó OG. Imatinib-induced fatal acute liver failure. World J Gastroenterol 2007; 13: 6608-111. PubMed Citation]
A 51 year old woman with chronic myelogenous leukemia (CML) developed fatigue and serum aminotransferase elevations 5 months after starting imatinib therapy (400 mg daily). Imatinib was discontinued, but her symptoms and liver tests worsened and she was admitted for evaluation two weeks later. She had no history of liver disease and serum enzymes had been normal before treatment (Table). She did not drink alcohol, had no risk factors for liver hepatitis and was not taking any other medications. Physical examination showed jaundice, but no signs of chronic liver disease or evidence of encephalopathy. At this point, serum bilirubin was 8.4 mg/dL, ALT 3185 U/L, AST 2224 U/L, and alkaline phosphatase 648 U/L. The prothrombin time index (protime) was 30%. Tests for hepatitis A, B and C were negative as were routine autoantibodies. Abdominal ultrasound showed the liver to be reduced in size, but without evidence of extrahepatic obstruction. There was mild ascites. Over the next few days, her clinical condition worsened, the prothrombin index fell to 6% and she developed hepatic encephalopathy. She was referred for liver transplantation, but required vasopressors and mechanical ventilation and died of multiorgan failure before a donor liver became available.
Key Points
| Medication: | Imatinib (400 mg daily) |
| Pattern: | Hepatocellular (R=30.5) |
| Severity: | 5+ (acute liver failure and death) |
| Latency: | 5 months |
| Recovery: | None |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | Pre | 55 | 197 | 0.5 | |
| 8 weeks | 13 | 330 | 0.3 | ||
| 18 weeks | 49 | 204 | 0.5 | ||
| 20 weeks | 0 | 1493 | 288 | 0.7 | Imatinib stopped |
| 22 weeks | 14 days | 3185 | 648 | 8.4 | Admission |
| 16 days | 3028 | 668 | 12.2 | Protime 30% | |
| 23 weeks | 21 days | 1941 | 831 | 25.4 | Protime 13% |
| 22 days | 1767 | 800 | 21.4 | Encephalopathy | |
| 24 weeks | 4 weeks | 900 | 686 | 24.7 | |
| Died of multiorgan failure | |||||
| Normal Values | <35 | <240 | <1.2 | ||
Comment
Despite prompt discontinuation of imatinib, this patient progressed to acute liver failure and died 4 weeks after onset of initial symptoms of liver injury and stopping therapy. In the first 10 years of imatinib availability, there have been over a dozen case reports of clinically apparent acute liver injury due to imatinib, and at least 6 instances of acute liver failure resulting in death or need for emergency liver transplantation.
Case 3. Severe reactivation of hepatitis B due to imatinib.
[Modified from: Kang BW, Lee SJ, Moon JH, Kim SN, Chae YS, Kim JG, Hwang YJ, Sohn SK. Chronic myeloid leukemia patient manifesting fatal hepatitis B virus reactivation during treatment with imatinib rescued by liver transplantation: case report and literature review. Int J Hematol 2009; 90: 383-7. PubMed Citation]
A 48 year old man with chronic myelogenous leukemia (CML) and the HBsAg carrier state developed nausea, weakness and jaundice accompanied by evidence of reactivation of hepatitis B, 9 months after starting imatinib. Before treatment, he was known to have normal serum aminotransferase levels and tested positive for HBsAg with low levels of HBV DNA (<2,000 copies/mL). In addition, he was HBeAg negative and anti-HBe positive. After the diagnosis of Philadelphia chromosome positive CML in the chronic phase, he was initially treated with hydroxyurea for 3 weeks and then switched to imatinib [400 mg daily]. Serum aminotransferase levels were monitored regularly (Table). Nine months after starting imatinib, he developed symptoms of liver disease and was found to be jaundiced. Laboratory tests showed total serum bilirubin of 8.3 mg/dL, ALT 254 U/L and HBV DNA of 100 million copies/mL. Tests for hepatitis A and C were negative as were tests for Epstein Barr virus and cyclomegalovirus infection. Ultrasonography showed no evidence of biliary obstruction, but did reveal an echogenic liver and mild splenomegaly. Despite stopping imatinib and starting lamivudine, his condition deteriorated with steady rise of serum bilirubin to a peak of 53 mg/dL, worsening prothrombin time and appearance of hepatic encephalopathy. A living donor liver transplantation was done approximately two months after presentation with jaundice. The explant showed a macronodular cirrhosis with superimposed submassive necrosis, inflammation and cholestasis. He recovered rapidly and was discharged within 6 weeks of surgery. In follow up, his serum bilirubin and enzymes remained normal and HBV DNA fell to undetectable levels. He remained on lamivudine and 7 months after transplantation he was restarted on imatinib without further complications.
Key Points
| Medication: | Imatinib (200 mg daily) |
| Pattern: | Probably hepatocellular |
| Severity: | 5+ (liver transplantation) |
| Latency: | 9 months |
| Recovery: | None |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | HBV DNA (copies/mL) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | Pre | 34 | <2000 | 0.9 | HBsAg positive |
| 0 | 30 | 0.9 | |||
| 3 month* | 60 | 0.7 | |||
| 6 months* | 70 | 1.0 | Imatinib stopped | ||
| 9 months | 0 | 254 | 100 million | 8.4 | Admission |
| 2 weeks | 280 | 22 | Protime 54% | ||
| 10 months | 4 weeks | 310 | 28 | ||
| 6 weeks | 160 | 43 | |||
| 11 months | 8 weeks | 90 | 53 | Protime 27% | |
| Living donor liver transplant performed | |||||
| 12 months | 13 weeks | 50 | 1.3 | Discharged | |
| 15 months | 6 months | 29 | <2000 | 0.5 | |
| 18 months | 10 months | 30 | 0.5 | Imatinib Restarted | |
| 22 months | 14 months | 30 | 0.8 | ||
| Normal Values | <40 | None | <1.2 | ||
*Several values and times are estimated from Figure 1.
Comment
Reactivation of chronic hepatitis B typically occurs in patients given corticosteroids or cancer chemotherapy who are HBsAg positive, but have minimal liver disease and low levels of HBV DNA and no detectable HBeAg in serum. The immune suppression causes an increase in HBV replication with a rise in HBV DNA to high levels; HBeAg may also reappear. Subsequent partial immune restoration is accompanied by an immunologic response to HBV antigens and a flare of the hepatitis B. Reactivation is well known to occur with cancer chemotherapy, particularly with high doses of corticosteroids or rituximab. Reactivation can also be caused by tumor necrosis factor antagonists such as imfliximab. The appearance of hepatitis B reactivation during therapy with imatinib, however, came as a surprise, as this therapy was not thought to cause significant immune suppression and was not suspected of altering HBV replication. Nevertheless, several very convincing instances of reactivation of hepatitis B have now been linked to imatinib therapy arising 3 months to more than a year after starting therapy. Reactivation of hepatitis B can be severe and has a mortality rate of at least 10%. While not shown to be effective in patients receiving imatinib, prophylaxis with antiviral agents has been shown to prevent or ameliorate hepatitis B reactivation after cancer chemotherapy and therefore can be recommended for patients with HBsAg in serum who are scheduled to receive imatinib therapy.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Imatinib – Gleevec®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Imatinib | 152459-95-5 | C29-H31-N7-O |
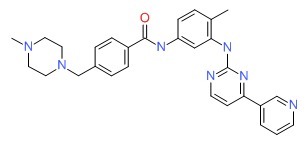 |
ANNOTATED BIBLIOGRAPHY
References updated: 24 April 2018
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of imatinib).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013. pp. 541-67.(Review of hepatotoxicity of cancer chemotherapeutic agents; discusses the interactions of imatinib with CYP 3A4; ALT elevations of >5 times ULN occur in 1-3% of patients on imatinib alone and in 53% of those receiving imatinib in combination with other agents; 5 reports of liver injury have been published including one case of acute liver failure).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagiv H, Sequist L, Wilson W, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-53.(Textbook of pharmacology and therapeutics).
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001; 344: 1031-7. [PubMed: 11287972](Phase 1 trial of imatinib in 83 patients with chronic myelogenous leukemia [CML]; side effects included nausea, myalgias, edema and rash; no mention of hepatotoxicity or ALT elevations).
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347: 472-80. [PubMed: 12181401](Randomized trial of 400 vs 600 mg of imatinib in 147 patients with gastrointestinal stromal tumors [GIST]; 54% had abnormal liver tests, but no details given).
- Elliott MA, Mesa RA, Tefferi A. Adverse events after imatinib mesylate therapy. N Engl J Med 2002; 346: 712-3. [PubMed: 11870257](Among 26 patients with malignancies treated with imatinib, 3 developed splenic rupture; all had splenomegaly and presented within 12-40 days with left upper quadrant pain requiring splenectomy).
- Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, Niederwieser D, et al.; International STI571 CML Study Group. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med 2002; 346: 645-52. [PubMed: 11870241](Open label study of imatinib in 532 patients with CML who had failed previous therapy; complete hematologic response occurred in 95% of patients; discontinuation for side effects was required in 2.1% and 4 patients had serious "hepatic toxic effects", but no details given).
- Schwetz BA. From the Food and Drug Administration. JAMA 2002; 287: 1103. [PubMed: 11879090](Announcement of approval of imatinib for unresectable or metastatic malignant gastrointestinal stromal tumors [GIST]).
- Ohyashiki K, Kuriyama Y, Nakajima A, Tauchi T, Ito Y, Miyazawa H, Kimura Y, et al. Imatinib mesylate-induced hepato-toxicity in chronic myeloid leukemia demonstrated focal necrosis resembling acute viral hepatitis. Leukemia 2002; 16: 2160-1. [PubMed: 12357373](56 year old woman with CML developed rash and fatigue 11 days after starting imatinib [bilirubin 3.8 mg/dL, ALT 342 U/L, Alk P 435 U/L, without fever or eosinophilia], resolving in 3 weeks).
- Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, Schiffer CA, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood 2002; 99: 3530-9. [PubMed: 11986204](Phase 2 trial of 400 or 600 mg of imatinib in 260 patients with CML in blast crisis; discontinuation for side effects occurred in 5% and was due to abnormal liver tests in one; ALT levels above 5 times ULN occurred in 2% and bilirubin >3 times ULN in 4%).
- Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, Schiffer CA, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 2002; 99: 1928-37. [PubMed: 11877262](Phase 2 trial comparing 400 vs 600 mg of imatinib in 235 patients with CML; ALT elevations >5 times ULN occurred in 3% and bilirubin in 2%; one death from acute liver failure within 12 days of starting imatinib).
- O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, et al.; IRIS Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348: 994-1004. [PubMed: 12637609](Controlled trial of imatinib vs interferon alfa and cytarabine in 1106 patients with CML; ALT elevations occurred in 43% of patients on imatinib and were above 5 times ULN in 5.1%, but no deaths from liver disease).
- Lin NU, Sarantopoulos S, Stone JR, Galinsky I, Stone RM, Deangelo DJ, Soiffer RJ. Fatal hepatic necrosis following imatinib mesylate therapy. Blood 2003; 102: 3455-6. [PubMed: 14568907](61 year old woman with polycytemia vera and myelofibrosis developed raised enzymes 7 weeks after starting imatinib [ALT 145 rising to 1041 U/L], with subsequent acute liver failure, lactic acidosis, shock and death; autopsy showing coagulative necrosis).
- James C, Trouette H, Marit G, Cony-Makhoul P, Mahon FX. Histological features of acute hepatitis after imatinib mesylate treatment. Leukemia 2003; 17: 978-9. [PubMed: 12750713](2 cases of marked ALT elevations [2430 and 159 U/L] in 58 and 35 year old women treated with imatinib for CML for 5 and 12 months, one with jaundice [bilirubin 21.9 mg/dL], resolving with stopping and recurring in both with restarting).
- Rocca P, El Jastimi S, Troncy J, Scoazec JY, Boucher A, Vial T, Trepo C, et al. [Imatinib mesylate-induced acute cytolytic hepatitis]. Gastroenterol Clin Biol 2004; 28: 918-9. French. [PubMed: 15523233](64 year old woman with CML developed ALT elevations 2 months after starting imatinib, with recurrence on restarting [bilirubin 5.7 mg/dL, ALT 28 times ULN, Alk P 3 times ULN], resolving in 3 months and recurring 2 weeks after restarting again).
- Kikuchi S, Muroi K, Takahashi S, Kawano-Yamamoto C, Takatoku M, Miyazato A, Nagai T, et al. Severe hepatitis and complete molecular response caused by Imatinib mesylate: possible association of its serum concentration and clinical outcomes. Leuk Lymphoma 2004; 45: 2349-51. [PubMed: 15512829](40 year old woman with CML developed jaundice 5 months after starting imatinib [bilirubin 2.7 rising to 10 mg/dL, ALT 559 U/L, prothrombin index 46%], resolving within 2 months of stopping).
- Ikuta K, Torimoto Y, Jimbo J, Inamura J, Shindo M, Sato K, Tokusashi Y, et al. Severe hepatic injury caused by imatinib mesylate administered for the treatment of chronic myeloid leukemia and the efficacy of prednisolone for its management. Int J Hematol 2005; 82: 343-6. [PubMed: 16298828](51 year old woman with CML developed fatigue 3 months after starting imatinib [bilirubin 14.0 mg/dL, ALT 167 U/L, Alk P 403 U/L, prothrombin index 61%], recurring upon restarting at low dose, but not recurring when given with prednisone [20 mg/day] which later was tapered and stopped).
- Ayoub WS, Geller SA, Tran T, Martin P, Vierling JM, Poordad FF. Imatinib (Gleevec)-induced hepatotoxicity. J Clin Gastroenterol 2005; 39: 75-7. [PubMed: 15599217](22 year old woman with CML developed jaundice 1 month after starting imatinib and after trip to Mexico [bilirubin 10.4 rising to 25.6 mg/dL, ALT 1337 U/L, Alk P 398 U/L], resolving 3 months after stopping).
- Cohen MH, Johnson JR, Pazdur R. U.S. Food and Drug Administration Drug Approval Summary: conversion of imatinib mesylate (STI571; Gleevec) tablets from accelerated approval to full approval. Clin Cancer Res 2005; 11: 12-9. [PubMed: 15671523](Summary of studies upon which FDA gave approval of imatinib for CML in chronic phase after failure of other therapy or accelerated phase or blast crisis; elevations in ALT >5 times ULN occurred in 2-4% of cases, and one patient [also taking acetaminophen] died of acute liver failure).
- Ferrero D, Pogliani EM, Rege-Cambrin G, Fava C, Mattioli G, Dellacasa C, Campa E, et al. Corticosteroids can reverse severe imatinib-induced hepatotoxicity. Haematologica 2006; 91 (6 Suppl): ECR27. [PubMed: 16785130](5 cases of ALT and AST elevations 3-8 months after starting imatinib for CML [ALT 183-1080 U/L, bilirubin and Alk P normal]; recurrence on restarting, but not if combined with prednisone, which allowed long term therapy).
- Zonder JA, Schiffer CA. Update on practical aspects of the treatment of chronic myeloid leukemia with imatinib mesylate. Curr Hematol Malig Rep 2006; 1: 141-51. [PubMed: 20425345](Review of use of imatinib in CML; hepatic toxicity occurs in 1-3%, but is rarely so severe to require drug discontinuation).
- Pariente A, Etcharry F, Cales V, Laborde Y, Ferrari S, Biour M. Imatinib mesylate-induced acute hepatitis in a patient treated for gastrointestinal stromal tumour. Eur J Gastroenterol Hepatol 2006; 18: 785-7. [PubMed: 16772838](Woman with GIST developed fatigue 6 weeks after starting imatinib [bilirubin 0.9 rising to 3.3 mg/dL, peak ALT 66 times ULN, Alk P 1.9 times ULN], resolving within 6 weeks of stopping and recurring rapidly on restarting [ALT 10 times ULN] even in lower doses [ALT 2.5 times ULN], later treated with sunitinib without recurrence).
- Dhalluin-Venier V, Besson C, Dimet S, Thirot-Bibault A, Tchernia G, Buffet C. Imatinib mesylate-induced acute hepatitis with autoimmune features. Eur J Gastroenterol Hepatol 2006; 18: 1235-7. [PubMed: 17033447](18 year old woman with CML developed asymptomatic serum enzyme elevations 2 weeks after starting imatinib [bilirubin 0.4 mg/dL, ALT 227 U/L, Alk P 74 U/L ], ALT rising [755 U/L with continuation and staying high [973 U/L] until prednisone was given [17 U/L]; positive rechallenge even with prednisone [137 U/L] and later switched to interferon: antiliver cytosol antibody was 1:640, but ANA negative).
- Cross TJ, Bagot C, Portmann B, Wendon J, Gillett D. Imatinib mesylate as a cause of acute liver failure. Am J Hematol 2006; 81: 189-92. [PubMed: 16493605](46 year old woman with CML had remission on long-term imatinib [18 months], but developed nausea a few weeks after stopping and restarting imatinib, progressing to acute liver failure [bilirubin 26 mg/dL, AST 201 U/L, Alk P 84 U/L, INR 2.8] and undergoing liver transplantation but dying postoperatively, explants showing confluent necrosis and collapse).
- Ikeda K, Shiga Y, Takahashi A, Kai T, Kimura H, Takeyama K, Noji H, et al. Fatal hepatitis B virus reactivation in a chronic myeloid leukemia patient during imatinib mesylate treatment. Leuk Lymphoma 2006; 47: 155-7. [PubMed: 16321842](54 year old man with CML and both chronic hepatitis B and C developed fatigue and jaundice 5 months after starting imatinib [bilirubin 5.6 rising to 25 mg/dL, ALT 1574 U/L, prothrombin index 14%, high levels of HBV DNA polymerase and no detectable HCV RNA], with progressive hepatic failure and death 5 weeks after stopping).
- Mindikoglu AL, Regev A, Bejarano PA, Martinez EJ, Jeffers LJ, Schiff ER. Imatinib mesylate (gleevec) hepatotoxicity. Dig Dis Sci 2007; 52: 598-601. [PubMed: 17219077](72 year old man with CML developed enzyme elevations [ALT 90 rising to 114 U/L] which worsened for 4 weeks after stopping [ALT 1894 U/L, Alk P 125 U/L, bilirubin 1.1 mg/dL] and peaked at 7-9 weeks [ALT 2200 U/L, bilirubin 9.0 mg/dL], thereafter decreasing to normal).
- Ridruejo E, Cacchione R, Villamil AG, Marciano S, Gadano AC, Mandó. Imatinib-induced fatal acute liver failure. World J Gastroenterol 2007; 13: 6608-111. [PMC free article: PMC4611306] [PubMed: 18161937](51 year old woman with CML developed weakness 5 months after starting imatinib [bilirubin 0.7 rising to 36 mg/dL, ALT 1493 U/L, Alk P 288 U/L], with progression to acute liver failure and death awaiting liver transplant 1 month after onset: Case 2).
- Kong JH, Yoo SH, Lee KE, Nam SH, Kwon JM, Lee SM, Chang HJ, et al. Early imatinib-mesylate-induced hepatotoxicity in chronic myelogenous leukaemia. Acta Haematol 2007; 118: 205-8. [PubMed: 18030002](64 year old man with CML developed rash, edema and dark urine 10 days after starting imatinib [bilirubin 18.9 mg/dL, ALT 610 U/L, Alk P 937 U/L, 12% eosinophils], resolving within 6 weeks; later restarted and had rashes, but no further hepatotoxicity).
- Fuster F, Medina L, Vallansot R, Granell M, Bruguera M. [Imatinib-induced toxic hepatitis: description of two cases and review of the literature]. Gastroenterol Hepatol 2007; 30: 525-30. [PubMed: 17980129](3 patients, ages 46-58 years old, treated with imatinib for CML developed ALT elevations at 4-8 months and resolving in 2 months; a third said to develop fatal acute liver failure, but few details given).
- Al Sobhi E, Zahrani Z, Zevallos E, Zuraiki A. Imatinib-induced immune hepatitis: case report and literature review. Hematology 2007; 12: 49-53. [PubMed: 17364993](17 year old woman with CML developed jaundice 1.5 years after starting imatinib [bilirubin 4.1 mg/dL, ALT 570 U/L, Alk P 336 U/L, ANA positive], improving on stopping imatinib and worsening again on restarting 3 weeks later, and then persisting for several months despite stopping, responding ultimately to prednisolone therapy).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, one case was attributed to imatinib).
- Lin AY, Fisher GA, So S, Tang C, Levitt L. Phase II study of imatinib in unresectable Hepatocellular carcinoma. Am J Clin Oncol 2008; 31: 84-8. [PubMed: 18376233](15 patients with unresectible hepatocellular carcinoma [7 with chronic hepatitis B] were treated with imatinib for up to 3 months; no patients had a tumor response, 9 had ALT elevations [but all <5 times ULN], few details given).
- Lakhani S, Davidson L, Priebat DA, Sherker AH. Reactivation of chronic hepatitis B Infection related to imatinib mesylate therapy. Hepatol Int 2008; 2: 498-9. [PMC free article: PMC2716906] [PubMed: 19669326](50 year old man with desmoid tumor and chronic HBsAg carrier state developed fatigue and rising ALT levels 3 months after starting imatinib with subsequent jaundice [peak bilirubin 7.1 mg/dL, ALT 2120 U/L, HBV DNA 29 million IU/mL, protime normal], resolving within 6 weeks of stopping with fall in HBV DNA levels [~3000 IU/mL]).
- Thia TJ, Tan HH, Chuah TH, Chow WC, Lui HF. Imatinib mesylate-related fatal acute hepatic failure in a patient with chronic myeloid leukaemia and chronic hepatitis B infection. Singapore Med J 2008; 49 (3): e86-9. [PubMed: 18362995](45 year old man with CML and chronic hepatitis B [HBV DNA 300,000 copies/mL] developed jaundice 5 months after starting imatinib [bilirubin 10.7 mg/dL, ALT 4193 U/L, Alk P 144 U/L, protime 25.9 sec, HBV DNA >100 million copies/mL], with rapidly evolving hepatic failure and death on hospital day 8).
- Kang BW, Lee SJ, Moon JH, Kim SN, Chae YS, Kim JG, Hwang YJ, Sohn SK. Chronic myeloid leukemia patient manifesting fatal hepatitis B virus reactivation during treatment with imatinib rescued by liver transplantation: case report and literature review. Int J Hematol 2009; 90: 383-7. [PubMed: 19641858](48 year old man with CML and chronic hepatitis B developed nausea followed by jaundice 9 months after starting imatinib [bilirubin 8.3 rising to 50 mg/dL, ALT 254 U/L, HBV DNA 100 million copies/mL, prothrombin index 54%], with worsening hepatic function despite stopping imatinib and starting lamivudine, ultimately undergoing successful liver transplant: Case 3).
- Al-Kali A, Farooq S, Tfayli A. Tumor lysis syndrome after starting treatment with Gleevec in a patient with chronic myelogenous leukemia. J Clin Pharm Ther 2009; 34: 607-10. [PubMed: 19744017](79 year old man with CML developed acute renal failure and tumor lysis syndrome within first week of starting imatinib, resolving with supportive care; no mention of hepatic involvement).
- Aliberti S, Grignani G, Allione P, Fizzotti M, Galatola G, Pisacane A, Aglietta M. An acute hepatitis resembling autoimmune hepatitis occurring during imatinib therapy in a gastrointestinal stromal tumor patient. Am J Clin Oncol 2009; 32: 640-1. [PubMed: 19955903](65 year old man with gastrointestinal stromal tumor developed raised ALT 1 month after starting imatinib [ALT peak 1056 U/L with normal bilirubin and Alk P], resolving in 4-5 weeks and patient was able to restart imatinib with prednisone therapy that was tapered and stopped after 6 months).
- Guilhot F, Druker B, Larson RA, Gathmann I, So C, Waltzman R, O'Brien SG. High rates of durable response are achieved with imatinib after treatment with interferon alpha plus cytarabine: results from the International Randomized Study of Interferon and STI571 (IRIS) trial. Haematologica 2009; 94: 1669-75. [PMC free article: PMC2791923] [PubMed: 19648168](Among 553 patients assigned to interferon and cytarabine therapy, 359 were crossed over to imatinib and treated for an average of 4 years, ALT or AST elevations occurred in 46% and were >5 times ULN in 4.7%).
- Perini GF, Santos FP, Funke V, Ruiz J, Neto BH, Hamerschlak N. Nilotinib post-liver transplantation for acute hepatic failure related to imatinib. Leuk Res 2009; 33: e234-5. [PubMed: 19632720](47 year old woman with CML developed jaundice and confusion after 18 months of imatinib therapy [bilirubin 20 mg/dL, ALT 828 U/L, prothrombin time 24 sec], leading to emergency liver transplantation; later treated with nilotinib without recurrence of liver injury).
- Tonyali O, Coskun U, Yildiz R, Karakan T, Demirci U, Akyurek N, Benekli M, et al. Imatinib mesylate-induced acute liver failure in a patient with gastrointestinal stromal tumors. Med Oncol 2010; 27: 768-73. [PubMed: 19662540](53 year old woman with gastrointestinal stromal tumor developed jaundice 10 weeks after starting imatinib [bilirubin 2.3 rising to 9.8 mg/dL, ALT 994 U/L, Alk P 195 U/L], treated with prednisolone with apparent response; after withdrawal of corticosteroids, liver tests remained normal; later treated with sunitinib: Case 1).
- Bilgi N, Bell K, Ananthakrishnan AN, Atallah E. Imatinib and Panax ginseng: a potential interaction resulting in liver toxicity. Ann Pharmacother 2010; 44: 926-8. [PubMed: 20332334](26 year old man with CML developed abdominal pain and liver test abnormalities 7 years after starting imatinib [bilirubin 1.4 mg/dL, ALT 1069 U/L, Alk P 124 U/L], having recently started ginseng, responding to prednisone therapy and stopping ginseng, ultimately able to restart imatinib).
- Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, Pasquini R, et al; ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251-9. [PubMed: 20525993](Controlled trial of 2 doses of nilotinib vs imatinib in 846 patients with CML found higher response rate with nilotinib [43% and 44% vs 22%]; although most side effects were less, ALT elevations were more frequent with nilotinib than imatinib [66% and 73% vs 20% for any elevation, 4% and 9% vs 2% for elevations >5 times ULN], hepatobiliary adverse events occurred in 4 nilotinib [0.7%] and 1 imatinib [0.4%] recipient; details not given).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, none of which were attributed to imatinib or other tyrosine kinase inhibitors).
- Kim SG, Chun JM, Jin R, Kim JY, Won DI, Hwang YJ. Living donor liver transplantation for acute hepatic failure caused by reactivation of hepatitis B virus infection after chemotherapy for hematologic malignancy: case reports. Transplant Proc 2010; 42: 843-5. (49 year old man with CML and cirrhosis due to hepatitis B developed jaundice, ascites and evidence of HBV reactivation. [PubMed: 20430187]more than a year after starting therapy with imatinib [peak bilirubin 50.8 mg/dL, HBV DNA 4.7 million copies/mL], undergoing successful emergency living donor liver transplantation).
- Spataro V. Nilotinib in a patient with postnecrotic liver cirrhosis related to imatinib. J Clin Oncol 2011; 29: e50-2. [PubMed: 20956624](41 year old woman with CML developed jaundice 6 months after starting imatinib [bilirubin 9.6 mg/dL, ALT 1374 U/L, Alk P 163 U/L, prothrombin index 27%, biopsy showing massive necrosis], recovering slowly with residual evidence of portal hypertension and subsequent biopsy showing cirrhosis; started on nilotinib for relapse in CML without worsening of liver tests).
- Gupta S, Bhatt VR, Varma S. Recurrent imatinib-induced hepatotoxicity in a chronic myeloid leukaemia patient successfully managed with prednisone. BMJ Case Rep 2011; 2011. (20 year old woman developed liver enzyme elevations 2 weeks after starting imatinib for CML [bilirubin 1.0 mg/dL, ALT 2364 U/L, Alk P 97 U/L], which resolved with stopping imatinib and recurred 1 week after restarting [bilirubin 2.5 mg/dL, ALT 654 U/L, Alk P 131 U/L], but not when it was restarted with prednisone which was later tapered and stopped). [PMC free article: PMC3062883] [PubMed: 22707550]
- Martínez Pascual C, Valdés Mas M, de la Peña Moral JM, Miras López M. [Fulminating hepatitis for imatinib in a patient with chronic myeloid leukaemia]. Med Clin (Barc) 2011; 137: 329-30. Spanish. [PubMed: 21074222](34 year old woman with CML developed jaundice 8 months after starting imatinib [bilirubin 14.5 mg/dL, ALT 1856 U/L, Alk P 254 U/L, prothrombin index 39%], progressing within weeks to hepatic failure requiring liver transplant; explant showed massive necrosis).
- García Hernández FJ, González León R, Castillo Palma MJ, Sánchez Román J. [Severe imatinib-induced hepatotoxicity]. Med Clin (Barc) 2012; 138: 638. Spanish. [PubMed: 22137996](Letter in response to Martinez Pascual [2011]).
- García-Valdés M, Miras López M, Garrido Corro B, De La Rubia Nieto A. [Survival following liver transplant due to imatinib-induced acute liver failure: a case study]. Farm Hosp 2012; 36: 50-1. Spanish. [PubMed: 21514865](34 year old woman developed jaundice 8 months after starting imatinib for CML [direct bilirubin 14.5 mgdL, ALT 1859 U/L, Alk P 254 U/L, prothrombin index 40%], with progressive liver failure and ultimately successful liver transplant).
- Wang YD, Cui GH, Li M, Gowrea B, Xia J, Hu Y. Hepatitis B virus reactivation in a chronic myeloid leukemia patient treated with imatinib mesylate. Chin Med J (Engl) 2012; 125: 2636-7. [PubMed: 22882953](40 year old man developed symptomatic reactivation of hepatitis B 6 months after starting imatinib for CML [bilirubin 3.0 mg/dL, ALT 1011 U/L, INR normal, HBV DNA 285,000 IU/mL], responding to entecavir and able to continue imatinib).
- Yachoui R. Early onset imatinib mesylate-induced hepatotoxicity in a patient with gastrointestinal stromal tumors. Am J Ther 2014; 21 (5): e148-50. (46 year old woman with gastrointestinal stomal tumors developed liver injury 11 days after starting imatinib, with resolution after stopping). [PubMed: 23567788]
- Lai GM, Yan SL, Chang CS, Tsai CY. Hepatitis B reactivation in chronic myeloid leukemia patients receiving tyrosine kinase inhibitor. World J Gastroenterol 2013; 19: 1318-21. [PMC free article: PMC3587491] [PubMed: 23483799](3 cases; 2 men and 1 woman, ages 43-67 years, developed reactivation of hepatitis B, 5, 15 and 53 months after starting imatinib (n=2] or nilotinib therapy of CML, with rise in ALT levels [374, 592 and 1086 U/L] and HBV DNA [2, 12 and 230 million IU/mL], but little or no jaundice [bilirubin normal, 2.54, and 2.74 mg/dL], and responding to entecavir therapy which allowed continuation of tyrosine kinase inhibitor).
- Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf 2013 36: 491-503. [PubMed: 23620168](Systematic review of the hepatotoxicity of tyrosine kinase inhibitors, most of which have been implicated in causing ALT elevations in a high proportion of patients and almost all of which have been implicated in cases of hepatitis, and some with fatal acute liver failure [crizotinib, imatinib, lapatinib, pazopanib, ponatinib, regorafenib, sunitinib]).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 1 attributed to imatinib during a period in which only 41 persons in Iceland were treated with imatinib).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 10 of which were attributed to antineoplastic agents including one due to imatinib).
- Mahon C, Purvis D, Laughton S, Bradbeer P, Teague L. Imatinib mesylate-induced pseudoporphyria in two children. Pediatr Dermatol 2014; 31: 603-7. [PubMed: 24920470](9 and 18 year old females developed blistering of the hands 2-3 years after starting oral imatinib [serum porphyrins normal] which resolved on stopping imatinib, the rash being considered part of the spectrum of cutaneous toxicity from kinase inhibitors, partially sunlight associated but not related to porphyrin metabolism).
- Yazici O, Sendur MA, Aksoy S. Hepatitis C virus reactivation in cancer patients in the era of targeted therapies. World J Gastroenterol 2014; 20: 6716-24. [PMC free article: PMC4051913] [PubMed: 24944464](Review of the effects of antineoplastic agents on hepatitis C and HCV RNA levels comments that there no reported cases of worsening on hepatitis C during imatinib therapy).
- Temel T, Gunduz E, Sadigova E, Uskudar Teke H, Meric Ozgenel S, Harmanci Ozakyol A. Hepatitis B virus reactivation under treatment with nilotinib. Euroasian J Hepatogastroenterol 2015; 5: 112-4. [PMC free article: PMC5578539] [PubMed: 29201705](59 year old woman with CML and HBsAg in serum [with HBeAg but no detectable HBV DNA] received imatinib for 11 months and 1 week after switching to nilotinib developed jaundice [bilirubin 4.0 mg/dL, ALT 42, Alk P not given, HBV DNA ~500,000 IU/mL], responding rapidly to tenofovir therapy and was able to restart nilotinib).
- Suzuki R, Kobayashi C, Sakai A, Fukushima H, Tagawa M, Satomi K, Nanmoku T, Sumazaki R, Fukushima T. Imatinib-induced severe hepatitis in a 9-year-old girl with Philadelphia chromosome-positive acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2015; 37: e368-71. [PubMed: 25929609](9 year old girl with Ph+ ALL developed worsening liver tests approximately 9 months after hematopoietic cell transplant and 14 weeks after restarting imatinib [bilirubin 1.1 rising to 9.4 mg/dL, ALT 973 U/L, Alk P not given], which progressed despite stopping imatinib but resolved after starting prednisolone, a later relapse was treated unsuccessfully with dasatanib).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 were attributed to antineoplastic agents [5.5%], 9 of which were attributed to kinase inhibitors, including 5 cases due to imatinib).
- Bunchorntavakul C, Reddy KR. Drug hepatotoxicity: newer agents. Clin Liver Dis 2017; 21: 115-34. [PubMed: 27842767](Review of the hepatotoxicity of recently approved medications including the tyrosine kinase inhibitors and imatinib which has been linked to serum enzyme elevations above 5 times ULN in 10-14% of patients and to individual cases of severe hepatotoxicity, some of which have been fatal).
- Bhatty O, Selim M, Kassim T, Chintalacheruvu L, Urra M, Shah S, Haggerty J, et al. A case of imatinib-induced hepatitis. Cureus 2017; 9: e1302. [PMC free article: PMC5493456] [PubMed: 28690936](71 year old woman with CML developed jaundice 6 months after starting imatinib [bilirubin 14.7 mg/dL, ALT 1816 U/L, Alk P 132 U/L, INR 2.1] with progressive liver failure despite stopping imatinib and starting prednisone, dying of multiorgan failure 1 month later).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Dasatinib.[LiverTox: Clinical and Researc...]Review Dasatinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ponatinib.[LiverTox: Clinical and Researc...]Review Ponatinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Bosutinib.[LiverTox: Clinical and Researc...]Review Bosutinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia.[Clin Ther. 2007]Review Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia.Steinberg M. Clin Ther. 2007 Nov; 29(11):2289-308.
- Review Nilotinib: a second-generation tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia.[Clin Ther. 2008]Review Nilotinib: a second-generation tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia.Deremer DL, Ustun C, Natarajan K. Clin Ther. 2008 Nov; 30(11):1956-75.
- Imatinib - LiverToxImatinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...