NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Omacetaxine is a semisynthetic cephataxine that acts as a protein translation inhibitor and is used to treated chronic myeloid leukemia that is resistant to tyrosine kinase receptor antagonists. Omacetaxine is associated with a low rate of serum enzyme elevation during therapy, but has not been linked to cases of clinically apparent liver injury with jaundice.
Background
Omacetaxine (oh" ma se tax' een) mepesuccinate is a semisynthetic molecule previously known as homoharringtonine which is derived from the leaves and bark of Cephalotaxus harringionia (plum yew). Omacetaxine is an inhibitor of protein translation which binds to ribosomes and blocks the initial elongation step in the synthesis of proteins from mRNA. Therapy with omacetaxine was found to have activity against chronic myeloid leukemia (CML) and was considered a first line agent before the introduction of the tyrosine kinase inhibitor imatinib. More recently, omacetaxine has found to improve survival in patients with refractory CML and resistance to multiple tyrosine kinase inhibitors and was approved for this use in the United States in 2012. Omacetaxine is administered parenterally and is available in single use vials of 3.5 mg of lyophilized powder for reconstitution. The recommended dose is 1.25 mg/m2 injected subcutaneously twice daily on days 1 to 14 of 28-day cycles, with subsequent modification once remission is achieved to twice daily on days 1 to 7 of 28-day cycles. Common side effects include myelosuppression which can be severe, injection site reactions, diarrhea, fatigue, weakness, nausea, headache, pyrexia and infections. Uncommon, but potentially severe adverse events include neutropenic fever and sepsis, cerebral hemorrhage and hyperglycemia.
Hepatotoxicity
In controlled trials, serum aminotransferase elevations occurred in 2% to 6% patients treated with omacetaxine, but most elevations were mild and transient with only rare patients requiring dose modification or discontinuation for liver test abnormalities. Clinically apparent liver injury with jaundice was not reported in the preregistration trials of omacetaxine and is not mentioned in the product label. Since the approval and more wide scale use of omacetaxine, there have been no publications or descriptions of the hepatotoxicity with jaundice associated with its use. Thus, clinically apparent liver injury due to omacetaxine must be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the liver enzyme elevations that occur during therapy with omacetaxine is unknown, but may be unrelated to the medication. Omacetaxine is metabolized mostly by circulating plasma esterases and has little hepatic metabolism. Omacetaxine exhibits no known drug-drug interactions.
Outcome and Management
The liver injury linked to omacetaxine therapy has been generally mild, consisting of transient and asymptomatic elevations in serum aminotransferase levels. Omacetaxine has not been linked to cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome. There is no reason to suggest that there is cross sensitivity to hepatic injury between omacetaxine and therapies of CML.
Drug Class: Antineoplastic Agents, Chronic Myeloid Leukemia Agents
Other Drugs in the Subclass, Chronic Myeloid Leukemia Agents: Bosutinib, Dasatinib, Imatinib, Nilotinib, Ponatinib
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Omacetaxine – Synribo®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Omacetaxine | 26833-87-4 | C29-H39-N-O9 |
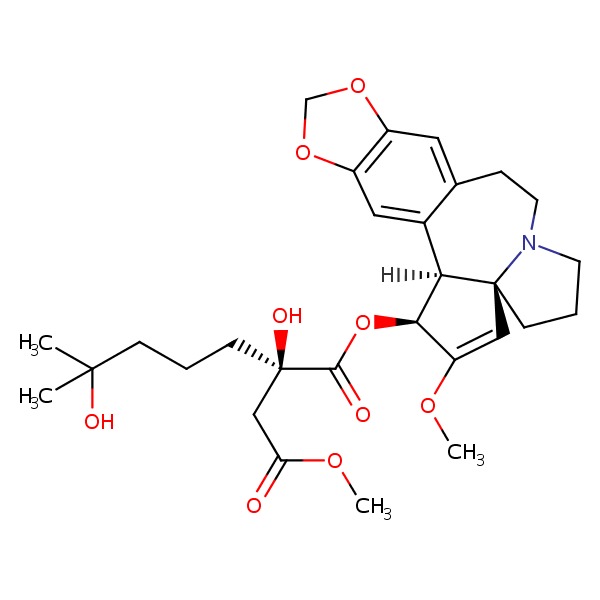 |
ANNOTATED BIBLIOGRAPHY
References updated: 06 January 2017
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999 before the availability of omacetaxine).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam, Elsevier, 2013, p. 541-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; omacetaxine is not discussed).
- Moy B, Lee RJ, Smith M. Hormone therapy in prostate cancer. Natural products in cancer chemotherapy: hormones and related agents. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1763-9.(Textbook of pharmacology and therapeutics).
- Cortes J, Lipton JH, Rea D, Digumarti R, Chuah C, Nanda N, Benichou AC, et al.; Omacetaxine 202 Study Group. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood 2012; 120: 2573-80. [PMC free article: PMC4916583] [PubMed: 22896000](Among 62 patients in the chronic phase of CML with a T351I mutation in the BCR-ABL gene and failure of imatinib therapy treated with omacetaxine [1-41 cycles], the hematologic response rate was 77% and side effects were largely due to myelosuppression; ALT elevations above 5 times ULN occurred in 2 patients [3%], but there were no liver related serious adverse events or discontinuations).
- Cortes J, Digumarti R, Parikh PM, Wetzler M, Lipton JH, Hochhaus A, Craig AR, et al.; Omacetaxine 203 Study Group. Phase 2 study of subcutaneous omacetaxine mepesuccinate for chronic-phase chronic myeloid leukemia patients resistant to or intolerant of tyrosine kinase inhibitors. Am J Hematol 2013; 88: 350-4. [PMC free article: PMC5558840] [PubMed: 23468307](Among 46 patients with CML in the chronic phase refractory to at least 2 tyrosine kinase inhibitors treated with omacetaxine [1 to 39 cycles], hematologic responses occurred in 67% and overall survival averaged 30 months, while side effects included thrombocytopenia [67%], anemia [54%], neutropenia [50%], infection [57%], diarrhea [44%], nausea [30%], fatigue [24%], and headache [20%]; there were no liver related serious adverse events, drug discontinuations or delays).
- Daver N, Vega-Ruiz A, Kantarjian HM, Estrov Z, Ferrajoli A, Kornblau S, Verstovsek S, et al. A phase II open-label study of the intravenous administration of homoharringtonine in the treatment of myelodysplastic syndrome. Eur J Cancer Care (Engl) 2013; 22: 605-11. [PMC free article: PMC3745781] [PubMed: 23701251](Among 9 patients with myelodysplasic syndromes treated with intravenous homoharringtonine [1-3 cycles], one had a complete response and 8 no response while side effects included serious cardiac and renal toxicities).
- Cortes JE, Nicolini FE, Wetzler M, Lipton JH, Akard L, Craig A, Nanda N, et al. Subcutaneous omacetaxine mepesuccinate in patients with chronic-phase chronic myeloid leukemia previously treated with 2 or more tyrosine kinase inhibitors including imatinib. Clin Lymphoma Myeloma Leuk 2013; 13: 584-91. [PMC free article: PMC3775895] [PubMed: 23787123](Among 108 patients with chronic phase CML who had received at least two tyrosine kinase inhibitors and were treated with omacetaxine in two phase 2 trials, the overall hematologic response rate was 69% and side effects including myelosuppression, diarrhea, nausea, fatigue, infections and fever; no mention of ALT elevations and of liver related serious adverse events).
- Kantarjian HM, O'Brien S, Cortes J. Homoharringtonine/omacetaxine mepesuccinate: the long and winding road to food and drug administration approval. Clin Lymphoma Myeloma Leuk 2013; 13: 530-3. [PMC free article: PMC3775965] [PubMed: 23790799](History of the development of omacetaxine as a therapy of CML; initially homoharringtonine, a natural plant alkaloid from Cephalotaxus trees, was studied extensively in China and reported to have activity against CML among other conditions; its cardiac toxicity and variability in purity led to semisynthetic modifications of the molecule [omacetaxine] and pharmacologically based dosing regimens and to its evaluation in cases of CML resistant to tyrosine kinase inhibitor therapy; future studies might focus on the combination of omacetaxine with tyrosine kinase inhibitors as first line therapy of CML).
- Alvandi F, Kwitkowski VE, Ko CW, Rothmann MD, Ricci S, Saber H, Ghosh D, et al. U.S. Food and Drug Administration approval summary: omacetaxine mepesuccinate as treatment for chronic myeloid leukemia. Oncologist 2014; 19: 94-9. [PMC free article: PMC3903068] [PubMed: 24309980](Description of the results of phase 2 open label studies of omacetaxine on which the FDA based an accelerated approval, but requesting follow up studies to document the duration of responses and studies of the safety of home preparation and administration of the subcutaneous injections).
- Khoury HJ, Cortes J, Baccarani M, Wetzler M, Masszi T, Digumarti R, Craig A, et al. Omacetaxine mepesuccinate in patients with advanced chronic myeloid leukemia with resistance or intolerance to tyrosine kinase inhibitors. Leuk Lymphoma 2015; 56: 120-7. [PubMed: 24650054](Among 95 patients with CML and resistance or intolerance of tyrosine kinase inhibitors who were treated with omacetaxine, 37% in the accelerated and 9% in blast phase had a hematologic response, and side effects were mostly due to myelosuppression; no mention of ALT elevations and no liver related serious adverse events).
- Cortes JE, Kantarjian HM, Rea D, Wetzler M, Lipton JH, Akard L, Khoury HJ, et al. Final analysis of the efficacy and safety of omacetaxine mepesuccinate in patients with chronic- or accelerated-phase chronic myeloid leukemia: Results with 24 months of follow-up. Cancer 2015; 121: 1637-44. [PMC free article: PMC5650096] [PubMed: 25586015](Among 122 patients with CML resistant or intolerant to tyrosine kinase inhibitors who were treated with omacetaxine in phase 2 trials, final follow up showed overall survival of 40 months in chronic phase and 14 months in accelerated phase cases; hematologic adverse events occurred in 79% and 73% of patients and led to early discontinuation in 10% and 5%; 69 patients [56%] had at least one serious adverse event, but none were liver related).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 [0.6%] were attributed to imatinib, but none to omacetaxine or other therapies of CML).
- Omacetaxine (Synribo) for CML. Med Lett Drugs Ther 2015; 57 (1469): e80-1. [PubMed: 26039555](Concise review of the mechanism of action, clinical efficacy, safety and costs of omacetaxine shortly after the FDA gave final approval for its use in adults with CML with resistance to tyrosine kinase inhibitors; no mention of hepatotoxicity or ALT elevations).
- Rosshandler Y, Shen AQ, Cortes J, Khoury HJ. Omacetaxine mepesuccinate for chronic myeloid leukemia. Expert Rev Hematol 2016; 9: 419-24. [PubMed: 26853281](Review of the development, mechanism of action, clinical efficacy and safety of omacetaxine; no mention of hepatotoxicity or ALT elevations).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review A Review of Omacetaxine: A Chronic Myeloid Leukemia Treatment Resurrected.[Oncol Ther. 2018]Review A Review of Omacetaxine: A Chronic Myeloid Leukemia Treatment Resurrected.Winer ES, DeAngelo DJ. Oncol Ther. 2018 Jun; 6(1):9-20. Epub 2018 Mar 15.
- Omacetaxine mepesuccinate--a semisynthetic formulation of the natural antitumoral alkaloid homoharringtonine, for chronic myelocytic leukemia and other myeloid malignancies.[IDrugs. 2008]Omacetaxine mepesuccinate--a semisynthetic formulation of the natural antitumoral alkaloid homoharringtonine, for chronic myelocytic leukemia and other myeloid malignancies.Quintás-Cardama A, Cortes J. IDrugs. 2008 May; 11(5):356-72.
- Review Omacetaxine Mepesuccinate for Chronic Myeloid Leukemia.[Expert Rev Hematol. 2016]Review Omacetaxine Mepesuccinate for Chronic Myeloid Leukemia.Rosshandler Y, Shen AQ, Cortes J, Khoury HJ. Expert Rev Hematol. 2016 May; 9(5):419-24. Epub 2016 Apr 21.
- Review Omacetaxine mepesuccinate for the treatment of chronic myeloid leukemia.[Drugs Today (Barc). 2013]Review Omacetaxine mepesuccinate for the treatment of chronic myeloid leukemia.Narayanan V, Gutman JA, Pollyea DA, Jimeno A. Drugs Today (Barc). 2013 Jul; 49(7):447-56.
- Review Omacetaxine mepesuccinate in the treatment of intractable chronic myeloid leukemia.[Onco Targets Ther. 2014]Review Omacetaxine mepesuccinate in the treatment of intractable chronic myeloid leukemia.Chen Y, Li S. Onco Targets Ther. 2014; 7:177-86. Epub 2014 Jan 31.
- Omacetaxine - LiverToxOmacetaxine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...