NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Boswellia also called Indian Frankincense is an extract of the gummy oleoresin derived from beneath the bark of the Boswellia serrata tree, which is native to India, the Middle East and Northern Africa. The resin is rich in triterpenic acids and has been used for centuries in traditional Ayurvedic medicine to treat inflammatory conditions. More recently, Boswellia serrata extracts have been marketed as helpful in arthritis, colitis and asthma. Extracts of Boswellia serrata have not been linked to serum aminotransferase elevations during treatment or to instances of clinically apparent acute liver injury.
Background
Boswellia serrata is a moderate sized deciduous tree that is native to India, the Middle East and Northern Africa. Stripping off the paper-thin bark of Boswellia trees reveals a gummy oleoresin, extracts of which have been used in Ayurvedic medicine for their antiinflammatory, antiseptic, astringent and stimulant activity to treat inflammatory conditions and gastrointestinal complaints. Boswellia extract is also used as a perfume and aroma (frankincense). Boswellia extract contains essential oils, terpenoids, sugars and volatile oils and prominently several pentacyclic triterpene acids such as beta boswellic acid. In vitro boswellic acid inhibits the synthesis of 5-lipooxygenase and decreases production of downstream pro-inflammatory mediators. Boswellia serrata extracts have been reported to be effective in alleviating symptoms of asthma, irritable bowel syndrome, inflammatory bowel disease and osteoarthritis, although the magnitude of these effects is not clear and Boswellia has not been approved for these uses in the United States. Boswellia serrata extracts are available over-the-counter in varying concentrations, the usual recommended dose being 250 to 500 mg two or three times daily. Boswellia serrata is also found in many multiingredient products advertised for joint health and gastrointestinal complaints. Side effects are few and largely mild and transient gastrointestinal symptoms of nausea, diarrhea or constipation. In most controlled studies, adverse events were no more frequent with Boswellia extracts than with placebo.
Hepatotoxicity
Boswellia serrata extract has not been linked to serum enzyme elevations during therapy, although there have been few prospective studies in humans that have reported on its effects on laboratory test results in any detail. In small trials, Boswellia extracts have appeared to be well tolerated with only minor and few adverse effects which have been similar in frequency among persons receiving placebo. Despite wide scale use as an herbal supplement, Boswellia extract has not been convincingly linked to published instances of clinically apparent liver injury. Boswellia is often included in multi-ingredient dietary supplements some of which have been implicated in liver injury, but a specific contribution from Boswellia to the injury could not be established. The frequency of hypersensitivity reactions to Boswellia is also not known.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Other Names: Indian frankincense, Indian oli-banum, Salai guggul and Sallaki.
Drug Class: Herbal and Dietary Supplements
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Boswellia Serrata – Generic
DRUG CLASS
Herbal and Dietary Supplements
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Boswellic Acid | 631-69-6 | C30-H48-O3 |
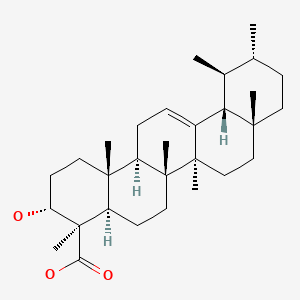
|
ANNOTATED BIBLIOGRAPHY
References updated: 03 November 2020
Abbreviation: HDS, herbal and dietary supplements.
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott,1999: pp. 731-4.(Expert review of hepatotoxicity published in 1999; several herbal medications linked to liver injury are discussed, but boswellia is not mentioned).
- Seeff L, Stickel F, Navarro VJ. Hepatotoxicity of herbals and dietary supplements. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 631-58.(Review of hepatotoxicity of herbals does not mention boswellia).
- PDR for herbal medicines. 4th ed. Montvale, New Jersey: Thomson Healthcare Inc. 2007.(Compilation of short monographs on herbal medications and dietary supplements does not discuss Boswellia serrata).
- Gupta I, Parihar A, Malhotra P, Singh GB, Lüdtke R, Safayhi H, Ammon HP. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur J Med Res. 1997;2:37–43. [PubMed: 9049593](Among 42 patients with ulcerative colitis treated with Boswellia gum resin [Salai guggal] or sulfasalazine for 6 weeks, clinical symptoms and histologic features improved in all and 18% on Boswellia had gastrointestinal side effects such as heartburn, anorexia, nausea or abdominal pain).
- Gupta I, Gupta V, Parihar A, Gupta S, Lüdtke R, Safayhi H, Ammon HP. Effects of Boswellia serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical study. Eur J Med Res. 1998;3:511–4. [PubMed: 9810030](Among 80 adults with asthma treated with Boswellia gum resin or placebo for 6 weeks, nearly all patients in both groups improved, but improvements in the Boswellia group were greater while only 2 subjects had side effects which were epigastric pain, hyperacidity and nausea).
- Gupta I, Parihar A, Malhotra P, Gupta S, Lüdtke R, Safayhi H, Ammon HP. Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med. 2001;67:391–5. [PubMed: 11488449](Among 30 patients with chronic colitis treated with Boswellia serrata extract [900 mg daily] or sulfasalazine [3 g daily] for 6 weeks, improvements occurred in 90% of Boswellia- vs 60% of sulfasalazine-treated subjects and side effects were “minimal”; heartburn reported in two subjects, but no mention of ALT elevations or hepatotoxicity).
- Gerhardt H, Seifert F, Buvari P, Vogelsang H, Repges R. Z Gastroenterol. 2001;39:11–7. [Therapy of active Crohn disease with Boswellia serrata extract H 15] German. [PubMed: 11215357](Among 83 patients with Crohn disease treated with Boswellia serrata extract or mesalazine for 8 weeks, changes in the Crohn Disease Activity Index with Boswellia were non-inferior to those with mesalazine and tolerance was excellent with no severe adverse events; no mention of ALT elevations or hepatotoxicity).
- Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee--a randomized double blind placebo controlled trial. Phytomedicine. 2003;10:3–7. [PubMed: 12622457](Among 30 patients with osteoarthritis of the knee treated with Boswellia extract or placebo for 8 weeks followed by crossover, all subjects had improvement in pain scores and walking distance on the herbal extract and adverse events were largely mild and transient gastrointestinal complaints; no mention of ALT elevations or hepatotoxicity).
- Chopra A, Lavin P, Patwardhan B, Chitre D. A 32-week randomized, placebo-controlled clinical evaluation of RA-11, an Ayurvedic drug, on osteoarthritis of the knees. J Clin Rheumatol. 2004;10:236–45. [PubMed: 17043520](Among 90 patients with osteoarthritis of the knee treated with a multiplant Ayurvedic product containing Boswellia serrata [with ashwagandha, ginger and curcumin] or a matched placebo for 32 weeks, pain scores improved more with the herbal supplement, and adverse events and changes in serum enzymes were mild and similar in the two groups).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, including 7 [5%] for herbal medications, none were specifically attributed to a product containing Boswellia).
- Langmead L, Rampton DS. Review article: complementary and alternative therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2006;23:341–9. [PubMed: 16422993](Review of complementary and alternative therapies for IBD mentions three trials of Boswellia serrata extracts in ulcerative colitis and Crohn disease, all of which reported moderate rates of remission, similar to standard therapies; no specific discussion of adverse events from Boswellia).
- Boswellia serrata. Monograph. Altern Med Rev. 2008;13:165–7. [PubMed: 18590352](Brief review of Boswellia serrata, extracts of the gummy oleoresin from the Indian tree that has been used for centuries as an antiinflammatory agent, expectorant, astringent and antiseptic agent in Ayurvedic medicine that contains essential oils, terpenoids, sugars and volatile acids including beta-boswellic acid which has been shown to inhibit 5-lipooxygenase and have antiinflammatory, anti-elastase and anti-apoptotic activities).
- García-Cortés M, Borraz Y, Lucena MI, Peláez G, Salmerón J, Diago M, Martínez-Sierra MC, et al. Rev Esp Enferm Dig. 2008;100:688–95. [Liver injury induced by "natural remedies": an analysis of cases submitted to the Spanish Liver Toxicity Registry] Spanish. [PubMed: 19159172](Among 521 cases of drug induced liver injury submitted to Spanish registry, 13 [2%] were due to herbals, but none were attributed to Boswellia).
- Jacobsson I, Jönsson AK, Gerdén B, Hägg S. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039–47. [PubMed: 19650152](Review of 778 spontaneous reports of adverse reactions to herbals to Swedish Registry; no mention of Boswellia).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 [11%] were attributed to drug induced liver injury of which 12 [9%] were due to herbals, but none were attributed to Boswellia).
- Holtmeier W, Zeuzem S, Preiss J, Kruis W, Böhm S, Maaser C, Raedler A, et al. Randomized, placebo-controlled, double-blind trial of Boswellia serrata in maintaining remission of Crohn's disease: good safety profile but lack of efficacy. Inflamm Bowel Dis. 2011;17:573–82. [PubMed: 20848527](Among 82 patients with Crohn disease in remission who were treated with Boswellia serrata extract or placebo for 52 weeks, the proportion of patients remaining in remission was similar in the two groups [60% vs 55%] as were rates of adverse events, and laboratory values showed no clinically significant changes in either group).
- Teschke R, Wolff A, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: a tabular compilation of reported cases. Liver Int. 2012;32:1543–56. [PubMed: 22928722](A systematic compilation of all publications on the hepatotoxicity of specific herbals identified 185 publications on 60 different herbs, herbal drugs and supplements, but Boswellia was not listed or mentioned).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a prospective database between 2004 and 2012, HDS were implicated in 145 [16%], the single major herbal cause being green tea and none were attributed to Boswellia [Navarro et al. Hepatology 2014]).
- García-Cortés M, Robles-Díaz M, Ortega-Alonso A, Medina-Caliz I, Andrade RJ. Hepatotoxicity by dietary supplements: A tabular listing and clinical characteristics. Int J Mol Sci. 2016;17:E537. pii. [PMC free article: PMC4848993] [PubMed: 27070596](Listing of published cases of liver injury from HDS products, but does not mention or list Boswellia).
- Brown AC. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem Toxicol 2017; 107(Pt A): 449-71. [PubMed: 27818322](Summary of the US regulations on safety and efficacy of herbal and dietary supplements).
- Brown AC. Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem Toxicol 2017; 107(Pt A): 472-501. [PubMed: 27402097](Description of an online compendium of cases of liver toxicity attributed to HDS products, does not mention or list Boswellia).
- Karlapudi V, Prasad Mungara AVV, Sengupta K, Davis BA, Raychaudhuri SP. A placebo-controlled double-blind study demonstrates the clinical efficacy of a novel herbal formulation for relieving joint discomfort in human subjects with osteoarthritis of knee. J Med Food. 2018;21:511–20. [PubMed: 29708818](Among 105 patients with osteoarthritis of the knee treated with a herbal preparation containing Boswellia serrata extract or placebo, scores for pain, quality of life and physical function improved with the herbal supplement and there were no serious adverse events or mention of ALT elevations or hepatotoxicity).
- Riva A, Giacomelli L, Togni S, Franceschi F, Eggenhoffner R, Zuccarini MC, Belcaro G. Oral administration of a lecithin-based delivery form of boswellic acids (Casperome®) for the prevention of symptoms of irritable bowel syndrome: a randomized clinical study. Minerva Gastroenterol Dietol. 2019;65:30–5. [PubMed: 30676012](Among 69 patients with mild forms of irritable bowel syndrome treated with Boswellia extract or placebo for 6 months, symptom scores for abdominal pain and altered bowel movements improved with the herbal extract and adverse events were mild and mostly “stipsis”; no mention of ALT elevations or hepatotoxicity).
- Majeed M, Majeed S, Narayanan NK, Nagabhushanam K. A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phytother Res. 2019;33:1457–68. [PMC free article: PMC6681146] [PubMed: 30838706](Among 48 patients with osteoarthritis of the knee treated with Boswellia extract or placebo for 120 days, symptoms of pain and stiffness were improved with Boswellia and there were no “clinically significant” changes in laboratory values).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Boswellia serrata, a potential antiinflammatory agent: an overview.[Indian J Pharm Sci. 2011]Boswellia serrata, a potential antiinflammatory agent: an overview.Siddiqui MZ. Indian J Pharm Sci. 2011 May; 73(3):255-61.
- Review Sustainable supply, a foundation for natural product development: The case of Indian frankincense (Boswellia serrata Roxb. ex Colebr.).[J Ethnopharmacol. 2018]Review Sustainable supply, a foundation for natural product development: The case of Indian frankincense (Boswellia serrata Roxb. ex Colebr.).Brendler T, Brinckmann JA, Schippmann U. J Ethnopharmacol. 2018 Oct 28; 225:279-286. Epub 2018 Jul 18.
- Simultaneous quantification of triterpenoic acids by high performance liquid chromatography method in the extracts of gum resin of Boswellia serrata obtained by different extraction techniques.[Chem Cent J. 2016]Simultaneous quantification of triterpenoic acids by high performance liquid chromatography method in the extracts of gum resin of Boswellia serrata obtained by different extraction techniques.Sharma N, Bhardwaj V, Singh S, Ali SA, Gupta DK, Paul S, Satti NK, Chandra S, Verma MK. Chem Cent J. 2016; 10:49. Epub 2016 Aug 4.
- Review Boswellia serrata: an overall assessment of in vitro, preclinical, pharmacokinetic and clinical data.[Clin Pharmacokinet. 2011]Review Boswellia serrata: an overall assessment of in vitro, preclinical, pharmacokinetic and clinical data.Abdel-Tawab M, Werz O, Schubert-Zsilavecz M. Clin Pharmacokinet. 2011 Jun; 50(6):349-69.
- Phytochemical Analysis and Anti-cancer Investigation of Boswellia serrata Bioactive Constituents In Vitro.[Asian Pac J Cancer Prev. 2015]Phytochemical Analysis and Anti-cancer Investigation of Boswellia serrata Bioactive Constituents In Vitro.Ahmed HH, Abd-Rabou AA, Hassan AZ, Kotob SE. Asian Pac J Cancer Prev. 2015; 16(16):7179-88.
- Boswellia Serrata - LiverToxBoswellia Serrata - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...