NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Bremelanotide is a parenterally administered melanocortin receptor agonist that is used to treat female hypoactive sexual desire disorder. Bremelanotide has been reported to cause mild serum enzyme elevations during therapy and has been implicated in rare instances of clinically apparent acute liver injury.
Background
Bremelanotide (bre” me lan’ oh tide) is a melanocortin receptor agonist that has been developed as a therapy of female hypoactive sexual desire disorder, which is defined as low sexual desire that causes marked distress or interpersonal difficulties. Melanocortin receptors are found largely in the central nervous system and skin and are associated with effects on appetite and sexual desire. In the skin, melanocortin receptors activate melanocytes and cause increase in pigmentation. Bremelanotide engages several melanocortin receptors (MCR), predominantly MC1R and MC4R, the latter of which is believed to modulate sexual desire. In several randomized, placebo controlled trials bremelanotide, given as a self-administered, on-demand subcutaneous injection at least 45 minutes before anticipated sexual activity, was found to improve indices of sexual desire and reduce sexual distress in a proportion of patients. Therapy did not consistently increase numbers of sexually satisfying events. Bremelanotide was approved for use in the United States in 2019 as therapy for premenopausal women with hypoactive sexual desire disorder. Bremelanotide is available in autoinjection pens as an solution of 1.75 mg in 0.3 mL under the brand name Vyleesi. The recommended dose is 1.75 mg subcutaneously by self-injection given at least 45 minutes before anticipated sexual activity. Common side effects include nausea (40%, particularly with the first injection), flushing (20% ), injection site reactions (13%) and headache (11%). Uncommon adverse reactions have included increase in blood pressure, severe nausea and vomiting, arthralgia, restless leg syndrome, and local hyperpigmentation.
Hepatotoxicity
In preregistration clinical trials, serum enzyme elevations were reported in small numbers of bremelanotide treated patients but in a similar proportion of subjects receiving placebo. A single case of acute hepatitis arose in a patient who had received 10 injections of bremelanotide over a one year period with marked elevations in serum aminotransferase levels, minimal increases in alkaline phosphatase, and mild jaundice which resolved after discontinuation. There have been no instances of acute liver failure or chronic liver injury attributed to bremelanotide, but the total clinical experience with this drug has been limited. Thus, acute liver injury from bremelanotide may occur but is rare.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which bremelanotide causes liver injury is not known. Bremelanotide is a synthetic peptide analogue of melanocyte stimulating hormone alpha (MSHα), and its metabolism is by hydrolysis of amide bonds and digestion by cellular peptidases. Bremelanotide has minimal drug-drug interactions and is unlikely to be inherently hepatotoxic or immunogenic.
Drug Class: CNS Stimulants; Female Hypoactive Sexual Desire Disorder Agents
See also Flibanserin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Bremelanotide – Vyleesi®
DRUG CLASS
Female Hypoactive Sexual Desire Disorder Agents
Product labeling at DailyMed, National Library of Medicine, NIH
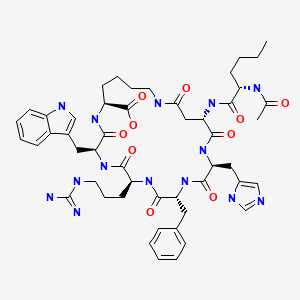
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
Bremelanotide | 189691-06-3 | C50-H68-N14-O10 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 August 2021
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Textbook of hepatotoxicity published in 1999, before the availability of bremelanotide).
- Schimmer BP, Funder JW. Adrenocorticotropic hormone, adrenal steroids, and the adrenal cortex. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 845-61.(Textbook of pharmacology and therapeutics).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/210557Orig1s000MultidisciplineR.pdf. (FDA website with product label and comprehensive multidiscipline review of the efficacy and safety of bremelanotide that supported its approval for use in female hypoactive sexual desire disorder, mentions that a single case of acute hepatitis occurred in a 52 year old woman who developed fatigue and weight loss approximately 10 days after receiving her last of 20 doses of bremelanotide with peak ALT 1163 U/L, Alk P 164 U/L and bilirubin 7.6 mg/dL, resolving over the next several months with normal values in follow up 1 to 2 years later [Simon 2019]). - Clayton AH, Althof SE, Kingsberg S, DeRogatis LR, Kroll R, Goldstein I, Kaminetsky J, et al. Bremelanotide for female sexual dysfunctions in premenopausal women: a randomized, placebo-controlled dose-finding trial. Womens Health (Lond). 2016;12:325–37. [PMC free article: PMC5384512] [PubMed: 27181790](Among 293 premenopausal women with hypoactive sexual desire disorder treated with self-administered, as desired injections of bremelanotide [0.75, 1.25 and 1.75 mg] or placebo for 12 weeks, the numbers of sexually satisfying events increased by 0.7 per month with bremelanotide vs 0.2 with placebo, and adverse events included nausea [18-24% vs 3%], flushing [14-17% vs 0], and headache [9-14% vs 3%], while “clinical laboratory findings showed no clinically significant trends”).
- Clayton AH, Lucas J, DeRogatis LR, Jordan R. Phase I randomized placebo-controlled, double-blind study of the safety and tolerability of bremelanotide coadministered with ethanol in healthy male and female participants. Clin Ther. 2017;39:514–526.e14. [PubMed: 28189361](Among 24 healthy adults given intranasal bremelanotide with or without alcohol or placebo on days 1, 4 and 7 in a three-way cross over study, side effects were similar in the 3 groups, although “a small number of participants had mild increases in ALT” of 1.5 to 3.0 times the upper limit of normal, one transiently rising to an ALT of 124 U/L, largely attributed to the alcohol).
- Bremelanotide (Vyleesi) for hypoactive sexual desire disorder. Med Lett Drugs Ther. 2019;61(1577):114–6. [PubMed: 31381550](Concise review of the mechanism of action, clinical efficacy, and safety of bremelanotide shortly after its approval in the US mentions side effects of nausea [40%], flushing, injection site reactions, headaches, and vomiting as well as transient increases in blood pressure and focal hyperpigmentation; no mention of ALT elevations or hepatotoxicity).
- Dhillon S, Keam SJ. Bremelanotide: First Approval. Drugs. 2019;79:1599–606. [PubMed: 31429064](Review of the structure, mechanism of action, history of development, pharmacokinetics, clinical efficacy and safety of bremelanotide shortly after its approval for use in the US mentions a case of acute icteric hepatitis that occurred in a patient who had received 8 doses of bremelanotide over a one year period in an open label extension study).
- Kingsberg SA, Clayton AH, Portman D, Williams LA, Krop J, Jordan R, Lucas J, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet Gynecol. 2019;134:899–908. [PMC free article: PMC6819021] [PubMed: 31599840](Among 1267 premenopausal women with hypoactive sexual desire disorder treated for 24 weeks with bremelanotide or placebo self-administered injections as needed before anticipated sexual activity, scores of sexual desire improved and those of sexual distressed decreased with bremelanotide and adverse reactions were common but generally mild-to-moderate in severity, and “no clinically significant effects on clinical laboratory tests…were observed”).
- Simon JA, Kingsberg SA, Portman D, Williams LA, Krop J, Jordan R, Lucas J, et al. Long-term safety and efficacy of bremelanotide for hypoactive sexual desire disorder. Obstet Gynecol. 2019;134:909–17. [PMC free article: PMC6819023] [PubMed: 31599847](Among 684 patients enrolled in an open-label extension study after participation in a 24 week controlled trial of bremelanotide of whom only 272 completed the extension period; while the authors state that “no new safety signals were observed”, one patient developed an acute hepatitis after receiving a year of therapy with serum aminotransferases rising to above 20 times ULN and bilirubin to twice ULN with minor elevations present six but not 12 months later).
- Kingsberg SA, Simon JA. Female hypoactive sexual desire disorder: a practical guide to causes, clinical diagnosis, and treatment. J Womens Health (Larchmt). 2020;29:1101–12. [PubMed: 32460605](Review of the pathogenesis, clinical features and therapy of female hypoactive sexual desire disorder).
- Spielmans GI. Re-analyzing Phase III bremelanotide trials for "hypoactive sexual desire disorder" in Women. J Sex Res. 2021:1–21. Epub ahead of print. [PubMed: 33678061](Critical analysis of the two controlled trials of bremelanotide [Kingberg 2019] in regard to changes in primary outcome during the course of analysis and the rather modest improvement in symptom scores).
- Kingsberg SA, Clayton AH, Portman D, Krop J, Jordan R, Lucas J, Simon JA. Failure of a meta-analysis: a commentary on Glen Spielmans's "Re-Analyzing Phase III Bremelanotide Trials for 'Hypoactive Sexual Desire Disorder in Women'". J Sex Res. 2021 Apr 9;:1–2. Epub ahead of print. [PubMed: 33835907](Reply to the critical analysis of Spielmans [2021] stressing the statistical rigor of the analysis and acceptance of the clinical endpoints used by the FDA).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Bremelanotide for Treatment of Female Hypoactive Sexual Desire.[Neurol Int. 2022]Review Bremelanotide for Treatment of Female Hypoactive Sexual Desire.Edinoff AN, Sanders NM, Lewis KB, Apgar TL, Cornett EM, Kaye AM, Kaye AD. Neurol Int. 2022 Jan 4; 14(1):75-88. Epub 2022 Jan 4.
- Bremelanotide for the Treatment of Hypoactive Sexual Desire Disorder: Two Randomized Phase 3 Trials.[Obstet Gynecol. 2019]Bremelanotide for the Treatment of Hypoactive Sexual Desire Disorder: Two Randomized Phase 3 Trials.Kingsberg SA, Clayton AH, Portman D, Williams LA, Krop J, Jordan R, Lucas J, Simon JA. Obstet Gynecol. 2019 Nov; 134(5):899-908.
- Bremelanotide: New Drug Approved for Treating Hypoactive Sexual Desire Disorder.[Ann Pharmacother. 2020]Bremelanotide: New Drug Approved for Treating Hypoactive Sexual Desire Disorder.Mayer D, Lynch SE. Ann Pharmacother. 2020 Jul; 54(7):684-690. Epub 2020 Jan 1.
- Prespecified and Integrated Subgroup Analyses from the RECONNECT Phase 3 Studies of Bremelanotide.[J Womens Health (Larchmt). 2022]Prespecified and Integrated Subgroup Analyses from the RECONNECT Phase 3 Studies of Bremelanotide.Simon JA, Kingsberg SA, Portman D, Jordan R, Lucas J, Sadiq A, Krop J, Clayton AH. J Womens Health (Larchmt). 2022 Mar; 31(3):391-400. Epub 2022 Feb 25.
- Review The neurobiology of bremelanotide for the treatment of hypoactive sexual desire disorder in premenopausal women.[CNS Spectr. 2022]Review The neurobiology of bremelanotide for the treatment of hypoactive sexual desire disorder in premenopausal women.Pfaus JG, Sadiq A, Spana C, Clayton AH. CNS Spectr. 2022 Jun; 27(3):281-289. Epub 2021 Jan 18.
- Bremelanotide - LiverToxBremelanotide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...