Copyright © 2024 - Canadian Agency for Drugs and Technologies in Health. Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International licence (CC BY-NC-ND).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Messages
What Is the Issue?
- Opioids are often used to help manage postoperative pain. However, their consumption can cause side effects, in addition to the risk of developing dependence with long-term use.
- Acetaminophen is an alternative analgesic that may provide opioid-sparing benefits for patients undergoing surgery (e.g., the need for patients to use opioids later), but there is a lack of synthesized evidence to confirm. Acetaminophen is available in different formulations, such as IV, oral, and rectal. However, there is uncertainty around the benefits of using 1 formulation over another perioperatively.
What Did We Do?
- To inform decisions about IV acetaminophen, we sought to identify and summarize literature comparing the effectiveness of IV acetaminophen to alternative analgesics (i.e., nonsteroidal anti-inflammatory drugs [NSAIDs]), alternative formulations (i.e., oral or rectal acetaminophen), or placebo for reducing opioid consumption in patients undergoing surgery.
- A research information specialist conducted a literature search of peer-reviewed and grey literature sources published between January 1, 2019, and January 9, 2024. The search was limited to English-language documents. One reviewer screened articles for inclusion based on predefined criteria. To investigate the true effect of IV acetaminophen, we excluded studies with any intraoperative opioid use.
What Did We Find?
- For adult patients undergoing elective hip surgery, there may be no significant differences in cumulative opioid use between postoperative IV and oral acetaminophen (1 randomized controlled trial).
- For patients undergoing elective cesarian delivery, postoperative IV acetaminophen may result in a decrease in total morphine consumption after surgery compared to placebo (1 randomized controlled trial).
- For adult patients undergoing lumbar disc surgery, there may be no significant differences in total morphine consumption for patients receiving intraoperative IV acetaminophen compared to placebo (1 systematic review with 1 relevant RCT).
- We did not find any studies comparing the opioid-sparing effects of IV acetaminophen to NSAIDs that met our criteria for this review.
What Does It Mean?
- Limited evidence from this review suggests that the opioid-sparing effect of IV acetaminophen may vary across types of surgery when compared to placebo. Additionally, IV acetaminophen may not offer additional opioid-sparing benefits compared to oral administration. However, we require more comprehensive research with rigorous methodological approaches to understand this topic better.
- Relative to opioids, IV acetaminophen has a preferable side effect profile, including a low risk of dependence; therefore, decision-makers may wish to consider using this formulation in the surgical or postsurgical setting.
Research Question
What is the clinical effectiveness of IV acetaminophen for opioid sparing (or reducing opioid consumption) in patients undergoing surgery?
Context and Policy Issues
Postoperative Pain Management
Postoperative pain is 1 of the most common complaints after surgery.1 Patients can feel postoperative pain immediately after surgery.1 Mechanical trauma during surgery activates the body’s nociceptive pathway responsible for the feeling of pain.1 A meta-analysis estimated the prevalence of moderate-to-severe postoperative pain 1 to 2 weeks after discharge to be up to 58%.2 Individuals with poorly managed postoperative pain can experience reduced quality of life, impaired physical function, and reduced sleep.1 Acute postoperative pain may also transition to chronic pain.1
Many clinicians rely on a multimodal peri-operative approach to manage acute pain from surgery.1,3 It involves non-pharmacological techniques, regional anesthesia, and pharmacotherapy, such as opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), and acetaminophen.1,3 A multimodal approach aims to minimize opioid use and its potential side effects.1,3 The short-term side effects of opioids include respiratory depression, excessive sedation, nausea, vomiting, urinary retention, and constipation, in addition to putting individuals at risk for opioid dependence and toxicity.3,4
Acetaminophen in Surgical Pain Management
Acetaminophen is 1 of the first-line agents used in multimodal pain management for its analgesic properties and its potential opioid-sparing effect or potential to reduce opioid consumption.1 Acetaminophen is available in different formulations, such as IV, oral, and rectal.1 Pharmacokinetic studies suggest that IV administration has improved analgesic effects compared to other formulations due to greater and faster blood-brain penetration and cerebrospinal bioavailability.5 Users may prefer the oral or rectal formulations because administering IV can cause some pain.1 However, there is limited evidence regarding the superiority of 1 formulation over another.1
Why Is This Issue Important?
The opioid crisis is an ongoing and growing concern globally and across Canada.6,7 The government of Canada reported over 40,000 apparent opioid-related deaths between January 2016 and June 2023.8 British Columbia, Alberta, and Ontario had the highest number of opioid-related deaths across jurisdictions in 2023.8
Surgery is a common indication for individuals to initiate opioid use.4 For example, 1 nonrandomized, retrospective study analyzed administrative health claims and found that up to 6.5% of adults living in the US develop new and persistent opioid use after surgery.9 In Canada, 3% of opioid-naive older adults reported prolonged opioid use, defined as an ongoing outpatient prescription for more than 90 days after a major elective surgery.10
Objective
The purpose of this report is to summarize and critically appraise the evidence regarding the clinical effectiveness of IV acetaminophen for opioid-sparing or reducing opioid consumption in patients undergoing surgery.
Methods
Literature Search Methods
An information specialist conducted a literature search on key resources including MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the International HTA Database, the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search approach was customized to retrieve a limited set of results, balancing comprehensiveness with relevancy. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. Search concepts were developed based on the elements of the research questions and selection criteria. The main search concepts were IV acetaminophen and opioid-sparing or reducing opioid consumption. CADTH-developed search filters were applied to limit retrieval to health technology assessments, systematic reviews, meta-analyses, or indirect treatment comparisons, and any types of clinical trials or observational studies. Conference abstracts were excluded. The search was completed on January 9, 2024 and limited to English-language documents published since January 1, 2019.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, 1 reviewer screened titles and abstracts and then retrieved potentially relevant articles to assess for inclusion. Table 1 presents the final selection of full-text articles based on the inclusion criteria.
Table 1
Selection Criteria.
Exclusion Criteria
We excluded articles if they did not meet the selection criteria outlined in Table 1, were duplicate publications, or were published before 2019. We excluded studies with confirmed intraoperative use of opioids, except when use was limited to surgical induction. We also excluded publications if we were unable to verify the absence of intraoperative opioid use.
Critical Appraisal of Individual Studies
One reviewer critically appraised the included publications using the following tools as a guide: A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2)11 for the systematic review and the Downs and Black checklist12 for the randomized studies. We did not calculate summary scores for the included studies; rather, the strengths and limitations of each included publication were described narratively.
Summary of Evidence
Quantity of Research Available
Appendix 1 presents study selection details. We identified 1 systematic review13 and 2 randomized controlled trials (RCTs)14,15 that addressed the clinical effectiveness of IV acetaminophen for opioid-sparing (or reducing opioid consumption) in patients undergoing surgery. Appendix 5 provides additional references of potential interest that did not meet the inclusion criteria.
Summary of Study Characteristics
Appendix 2 provides detailed characteristics of the included publications.
We identified 1 systematic review13 and 2 RCTs14,15 regarding the clinical effectiveness, specifically the opioid-sparing effects, of IV Acetaminophen compared to relevant comparators.
Systematic Review
The systematic review compared the impact of peri-operative IV acetaminophen to placebo on total morphine consumption within 24 hours after lumbar disc surgery.13 The systematic review authors searched 5 databases, as well as grey literature, for studies published up to October 2021. They also updated their search before submitting their review for publication. Ultimately, they included 5 RCTS.13 Of the 5, 1 RCT published in 2014 by Shimia and colleagues,16 met the inclusion criteria of this report. The 4 ineligible RCTs reported intraoperative use of opioids.13 As reported by the systematic review, the RCT by Shimia and colleagues16 compared the opioid-sparing effects of 1g of acetaminophen in 100 mL (i.e., paracetamol) to placebo (i.e., 100 mL of 0.9% sodium chloride). Both study arms received their assigned treatment within the last 20 minutes of surgery.13,16
Primary Studies
We identified 2 primary studies relevant to this report.14,15 Both studies used a single-centre, double-blinded, RCT design.14,15 The RCTs differed in their study populations, specifically by type of surgery. Namely, 1 RCT included women undergoing elective Caesarean delivery in Turkey,15 whereas the other included adults undergoing hip surgery in the US.14 It is important to note that Aksoy and colleagues15 referred their study population as women. We acknowledge that gender is a spectrum, and such language is not inclusive to trans and nonbinary persons. When study investigators used the term women in the study, we retained these terms in our reporting.
Both RCTs administered 1 g of IV acetaminophen in 100 mL solution to their acetaminophen treatment arms.14,15
The study by Aksoy et al.15 focused on patients undergoing elective Caesarean delivery compared IV paracetamol to placebo (i.e., subcutaneous 20 mL and IV saline) delivered after surgery. They evaluated total morphine consumption through patient-controlled analgesia after Caesarean delivery without a clear description of the length of follow-up.15
The RCT by Westrich et al.14 focused on adults undergoing hip surgery compared the opioid-sparing effects between IV and oral formulations of acetaminophen. To ensure adequate blinding, study investigators provided oral placebo to participants in the IV treatment arm, whereas they provided an IV placebo to individuals in the oral treatment arm.14 The study investigators quantified cumulative opioid use measured as opioid consumption in milligrams of oral morphine equivalent during the first 3 days (or 72 hours) after hip surgery.14
We did not identify studies comparing the opioid-sparing effects of acetaminophen to NSAIDs.
Summary of Critical Appraisal
Appendix 3 presents additional details regarding the strengths and limitations of included publications.
Systematic Review
The systematic review by Yin et al. (2022)13 clearly described its objectives and eligibility criteria. The study authors registered the review protocol in PROSPERO and used a comprehensive search strategy without language or publication date restrictions. However, they limited their inclusion criteria to RCTs without justification and did not provide a list of excluded studies, indicating potential selection bias. They used 2 independent reviewers to screen publications for inclusion, extract data, and assess risk of bias (using tools by the Cochrane Collaboration).
The authors deviated from the protocol without justification: the protocol specified that the interventions of interest included both oral and IV acetaminophen, but IV paracetamol was the intervention of interest reported in the published systematic review (i.e., oral paracetamol was not listed in their research question or eligibility criteria). Similarly, the authors did not conduct the planned subset analysis based on age or share the results as outlined in the protocol. These examples may suggest potential reporting bias.
The authors included studies with low and unclear risk of bias, and it appears the authors performed analyses to investigate the impact of risk of bias. They likely used appropriate methods to combine data for meta-analysis. For example, the authors used a random-effects model to analyze data for the relevant outcome and account for the considerable heterogeneity between included studies. The Begg’s test and Egger’s test found no risk of publication bias.
The external validity of the review is unclear. The study authors conducted the systematic review in China, and the investigators of 1 relevant RCT conducted their study in Iran. The authors reported no commercial or financial conflicts of interest but did not disclose whether they received any funding to conduct the systematic review.13
Primary Studies
The study investigators of the 2 included RCTs detailed their objectives or hypotheses, intervention of interest, and eligibility criteria.14,15 The study investigators of both RCTs recruited their participants from the same centre over the same period. Study authors of both RCTs sufficiently powered their study based on sample size calculations. The study investigators randomly allocated participants to treatment arms, while ensuring adequate blinding of participants as well as the clinical and research personnel involved in the study. Both RCTs reliably demonstrated compliance across study arms. We did not identify any unplanned or ad-hoc analysis from either RCTs.14,15
The RCT by Aksoy et al.15 clearly reported baseline patient characteristics and did not have any participants lost to follow up. Study investigators did not clearly describe the length of follow up time for total morphine consumption outcome. Hence, it is unclear if investigators followed participants for the same amount of time across and within treatment arms, indicating potential measurement bias. We also found limitations to suggest reporting bias in the results. The study investigators indicated when P values were < 0.05 in comparing 2 treatment arms, but they did not report actual P values. Additionally, the study investigators did not consider the impact of potential confounders on opioid consumption, such as baseline pain before postoperative pain management or previous Caesarean deliveries that can influence pain perception. We are unclear if these confounding factors impacted the study results since investigators did not report their distribution across study participants. The study investigators did not report any details about adverse events. Aksoy et al.15 declared no conflicts of interest, nor did the authors receive funding.
The RCT by Westrich et al.14 clearly described the baseline characteristics of participants, including potential confounding factors, such as baseline pain at rest and ambulation, race and ethnicity, and surgical time. However, the study investigators did not define the range of the numerical rating scale used (i.e., the upper limit of the scale) to measure participants’ baseline pain scores at rest and with ambulation. They included all the necessary details in the results section (e.g., all P values, simple outcome data, effect estimates). Study investigators did not describe the characteristics of participants lost to follow-up; however, it appears they handled losses to follow-up appropriately by performing a multiple imputation sensitivity analysis, which they described as producing a similar result. They also found that race and surgical time differed between treatment arms, but they did not address its potential impact to the findings. The PRISMA flow diagram reported that study investigators excluded individuals based on physician judgement, but the report did not provide any details about this criterion for exclusion. Hence, selection bias may be of concern in the recruitment of this study. Their hospital’s Special Surgery Research and Education Fund and Mallinckrodt Pharmaceutical, which also produced the intervention drug used, funded the study.14
Both studies took place in a single centre outside of Canada.14,15 We could not determine if individuals asked to participate in both RCTs were representative of the population from which they were recruited. Hence, results may not be valid or generalizable to centres or hospitals outside where studies were conducted.
Summary of Findings
Appendix 4 presents the main study findings from the 3 relevant publications.
IV Acetaminophen Versus Oral Acetaminophen
One RCT compared the opioid-sparing effects of postoperative IV acetaminophen to oral acetaminophen in adults who underwent elective hip surgery.14 The primary and sensitivity analyses found no statistically significant differences between the IV and oral formulations of postoperative acetaminophen on cumulative opioid use between day 0 and 3 after surgery.14
IV Acetaminophen Versus Placebo
The relevant RCT16 from the included systematic review by Yin et al.13 found intraoperative acetaminophen (paracetamol) resulted in less total morphine consumption 24 hours after surgery compared to placebo. However, this numerical difference was not statistically significant.13 The RCT conducted by Aksoy and colleagues15 found a statistically significant decrease in total morphine consumption for the postoperative IV acetaminophen group compared to the placebo. However, the length of follow up is unclear.15
Limitations
Quantity and Quality of Evidence
We found limited evidence (1 systematic review and 2 RCTs) about the opioid-sparing effects of IV acetaminophen. We identified studies specific to adults undergoing lumbar disc surgery,13 elective Caesarean delivery,15 and elective hip surgery.14 Hence, the results of these studies may not translate to other types of surgery or pediatric patients undergoing surgery. The quantity of evidence is further limited when considering the evidence by specific comparator. We did not identify studies comparing the opioid-sparing effects of acetaminophen to NSAIDs; therefore, we cannot form conclusions for this comparison. We identified 1 study that compared IV acetaminophen to the oral formulation14 and 2 studies that compared IV acetaminophen to placebo.13,15 Hence, we could not draw substantive conclusions about IV acetaminophen’s clinical effectiveness, specifically its potential to reduce opioid consumption. The lack of evidence may be linked to the common practice of multimodal analgesia perioperatively, such as opioids plus non-opioid analgesia, including IV acetaminophen.1,3 However, this report aimed to identify the true effect of IV acetaminophen on postoperative opioid consumption; therefore, we excluded studies that used opioids during surgery for either study arm.
The systematic review comparing IV acetaminophen and placebo identified 5 RCTs of low-to-moderate quality. Of the 5, 1 RCT was eligible for inclusion in this report. The systematic review authors determined that the risk of bias in this relevant RCT was unclear.
External Validity
The systematic review (SR) was conducted by authors from China, and the 1 relevant study within the SR16 was conducted in a surgical centre in Iran.13 Similarly, both RCTs were conducted in surgical centres outside of Canada (i.e., Turkey, US).14,15 Peri-operative pain management and surgical approach may differ from those provided for individuals in Canada for the same indications. Hence, it is unclear whether these findings are applicable to surgical centres operating in Canada.
These limitations warrant taking caution when interpreting the findings of this review.
Conclusions and Implications for Decision- or Policy-Making
This review identified and summarized the evidence available (1 systematic review with 1 relevant RCT,13 2 RCTs14,15) on the clinical effectiveness of IV acetaminophen for reducing opioid consumption in patients undergoing surgery.
We did not identify any relevant studies addressing the clinical effectiveness of IV acetaminophen compared with NSAIDs for patients undergoing any type of surgery. The limited evidence identified suggests that IV acetaminophen may not offer decreased opioid consumption postoperatively when compared to:
- oral acetaminophen for adult patients undergoing elective hip surgery14 or
- placebo for adult patients undergoing lumbar disc surgery.13
For patients undergoing elective cesarian delivery,15 postoperative IV acetaminophen may result in a decrease in total morphine consumption after surgery compared to placebo. Though, the study authors did not provide context about the length of follow-up time for this outcome.15
Decision-makers may use this report to understand the evidence to inform the use in the surgical or postsurgical setting with the important caveat that these results are from the limited amount of literature identified. The included studies focused on 3 different surgical populations, and we did not find published evidence regarding other surgical populations that met the eligibility criteria for this review. All evidence identified focused on adult populations living outside of Canada, which does not tell us how IV acetaminophen might work for pediatric populations or within Canada’s health care system.
To examine the true effect of acetaminophen, we excluded any study with intraoperative opioids. Future researchers may consider expanding the eligibility criteria to include studies with intraoperative or postoperative acetaminophen as an adjunct to intraoperative opioids, which may better reflect real-world application.17-19 In addition, decision-makers may consider whether IV acetaminophen has an effect on other outcomes relevant to surgical pain management, such as subjective pain scores or opioid-related side effects, which also affects patient experience and quality of life after surgery.
A 2023 CADTH Health Technology Review explored the use of IV acetaminophen in the Emergency Department. This report stated IV acetaminophen may offer similar levels of pain relief and a similar risk of adverse events as oral acetaminophen or IV NSAIDs for adults with moderate-to-severe pain (evidence from 1 SR and 2 RCTs).20 We identified additional studies that compared IV acetaminophen to alternate analgesics for patients in the intensive care unit, listed in Appendix 5: we excluded these studies because they were not specific to patients undergoing surgery. Together, these studies may provide a broader understanding of the clinical effectiveness of IV acetaminophen for patient populations outside the scope of this report.
In conclusion, limited evidence suggests that the opioid-sparing effect of IV acetaminophen varies depending on the surgery type compared with placebo and IV acetaminophen may not offer additional opioid-sparing benefits compared to oral acetaminophen. We require more comprehensive research with rigorous methodological approaches to understand this topic better.
Abbreviations
- AMSTAR 2
A MeaSurement Tool to Assess systematic Reviews 2
- MeSH
Medical Subject Headings
- NSAID
nonsteroidal anti-inflammatory drug
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- RCT
randomized controlled trial
Contributors: Chris Kamel, Thyna Vu
References
- 1.
- Mazda Y, Jadin S, Khan JS. Postoperative pain management. Canadian Journal of General Internal Medicine. 2021;16(S1):5-17. https://www
.utpjournals .press/doi/abs/10 .22374/cjgim.v16iSP1.529. Accessed 2024 Jan 12. - 2.
- Park R, Mohiuddin M, Arellano R, Pogatzki-Zahn E, Klar G, Gilron I. Prevalence of postoperative pain after hospital discharge: Systematic review and meta-analysis. Pain Rep. 2023;8(3):e1075. [PMC free article: PMC10168527] [PubMed: 37181639]
- 3.
- Mariano E. Approach to the management of acute pain in adults. In: Maniker R, ed. UpToDate. Waltham (MA): UpToDate; 2023: https://www
.uptodate.com. Accessed 2024 Jan 18. - 4.
- Hah J, Khan J, Nwaneshiudu C. Risk of long term opioid use and misuse after prescription of opioids for pain. In: Maniker R, ed. UpToDate. Waltham (MA): UpToDate; 2023: https://www
.uptodate.com. Accessed 2024 Jan 18. - 5.
- Singla NK, Parulan C, Samson R, et al. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012;12(7):523-532. [PubMed: 22524979]
- 6.
- United Nations Office on Drugs and Crime. Understanding the global opioid crisis. Global SMART Update 2019;21. https://www
.unodc.org /documents/scientific /Global_SMART_21_web_new.pdf. Accessed 2024 Jan 30. - 7.
- Belzak L, Halverson J. The opioid crisis in Canada: A national perspective. Health Promot Chronic Dis Prev Can. 2018;38(6):224-233. [PMC free article: PMC6034966] [PubMed: 29911818]
- 8.
- Government of Canada. Opioid- and stimulant related harms in Canada. 2023; https:
//health-infobase .canada.ca/substance-related-harms /opioids-stimulants/. Accessed 2024 Feb 5. - 9.
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. [PMC free article: PMC7050825] [PubMed: 28403427]
- 10.
- Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: Population based cohort study. BMJ. 2014;348:g1251. [PMC free article: PMC3921439] [PubMed: 24519537]
- 11.
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [PMC free article: PMC5833365] [PubMed: 28935701]
- 12.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. [PMC free article: PMC1756728] [PubMed: 9764259]
- 13.
- Yin F, Wang XH, Liu F. Effect of intravenous paracetamol on opioid consumption in multimodal analgesia after lumbar disc surgery: A meta-analysis of randomized controlled trials. Front Pharmacol. 2022;13:860106. [PMC free article: PMC9168366] [PubMed: 35677452]
- 14.
- Westrich GH, Birch GA, Muskat AR, et al. Intravenous vs oral acetaminophen as a component of multimodal analgesia after total hip arthroplasty: A randomized, blinded trial. J Arthroplasty. 2019;34(7S):S215-S220. [PubMed: 30948288]
- 15.
- Aksoy H, Ak M, Gokahmetoglu G, Aksoy U. The comparison of intraincisional bupivacaine infiltration and intravenous paracetamol administration for pain alleviation after cesarean section: A double-blinded randomized placebo controlled clinical trial. Eur Rev Med Pharmacol Sci. 2023;27(8):3467-3474. [PubMed: 37140296]
- 16.
- Shimia M, Parish M, Abedini N. The effect of intravenous paracetamol on postoperative pain after lumbar discectomy. Asian Spine J. 2014;8(4):400-404. [PMC free article: PMC4149981] [PubMed: 25187855]
- 17.
- Iyer S, Steinhaus ME, Kazarian GS, et al. Intravenous ketorolac substantially reduces opioid use and length of stay after lumbar fusion: A randomized controlled trial. Spine (Phila Pa 1976). 2024;49(2):73-80. [PMC free article: PMC10872662] [PubMed: 37737686]
- 18.
- Surgery and opioids: Best practice guidelines 2021. London (GB): Faculty of Pain Medicine; 2021: https://fpm
.ac.uk/sites /fpm/files/documents /2021-03/surgery-and-opioids-2021_4 .pdf. Accessed 2024 Jan 30. - 19.
- Verret M, Lam NH, Fergusson DA, et al. Intraoperative pharmacologic opioid minimisation strategies and patient-centred outcomes after surgery: A scoping review protocol. BMJ Open. 2023;13(3):e070748. [PMC free article: PMC9980324] [PubMed: 36858477]
- 20.
- Brett K, Severn M. CADTH health technology review: IV acetaminophen for acute pain in emergency departments. Can J Health Technol. 2023;3(10). https://www
.canjhealthtechnol .ca/index.php /cjht/article/view/RC1508/RC1508. Accessed 2024 Feb 5. [PubMed: 38096351]
Appendix 1. Selection of Included Studies
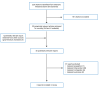
Figure 1
Selection of Included Studies.
Appendix 2. Characteristics of Included Publications
Note that this appendix has not been copy-edited.
Table 2
Characteristics of the Included Systematic Review.
Table 3
Characteristics of Included Randomized Controlled Trials.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has not been copy-edited.
Table 4
Strengths and Limitations of Systematic Review Using AMSTAR 211.
Table 5
Strengths and Limitations of Clinical Studies Using the Downs and Black Checklist.
Appendix 4. Main Study Findings
Note that this appendix has not been copy-edited.
Table 6
Summary of Findings by Comparison — IV Acetaminophen Versus Oral Acetaminophen.
Table 7
Summary of Findings by Comparison — IV Acetaminophen Versus Placebo.
Appendix 5. References of Potential Interest
- Brett K, Severn M. CADTH Health technology review: IV acetaminophen for acute pain in emergency departments. Can J Health Technol. 2023;3(10). https://www
.canjhealthtechnol .ca/index.php /cjht/article/view/RC1508/RC1508 [PubMed: 38096351] - Fillingham YA, Hannon CP, Erens GA, et al. The efficacy and safety of acetaminophen in total joint arthroplasty: Systematic review and direct meta-analysis. J Arthroplasty. 2020;35(10):2715-2729. [PubMed: 32563592]
- Hilleman DE, Malesker MA, Aurit SJ, Morrow L. Evidence for the efficacy of an opioid-sparing effect of intravenous acetaminophen in the surgery patient: A systematic review. Pain Med. 2020;21(12):3301-3313. [PubMed: 32869091]
- Archer VA, Samiee-Zafarghandy S, Farrokyhar F, Briatico D, Braga LH, Walton JM. Intravenous acetaminophen for postoperative pain in the neonatal intensive care unit: A protocol for a pilot randomized controlled trial (IVA POP). PLoS One. 2023;18(11):e0294519. [PMC free article: PMC10659208] [PubMed: 37983228]
- Higashitsuji A, Tomioka Y, Tanabe T, Ami N, Tei I. Intravenous acetaminophen reduces the length of intubation and rescue analgesics in intensive care unit patients after cardiovascular surgery in Japan: A retrospective analysis. J Opioid Manag. 2023;19(4):291-299. [PubMed: 37644787]
- Torres CM, Geneslaw AS, Svoboda L, Smerling AJ, Schlosser Metitiri KR. Effect of standing intravenous acetaminophen on postoperative opioid exposure in a pediatric cardiac intensive care unit. J Pediatr. 2023;255:236-239.e232. [PubMed: 36572175]
- Taylor BM, Chakraborty SR, Harthan AA, Tripathi S, Wang H, Swayampakula AK. Effect of IV acetaminophen usage on opioid requirements, outcomes and costs of care for postoperative children in a pediatric intensive care unit. J Pediatr Pharmacol Ther. 2020;25(6):514-520. [PMC free article: PMC7439945] [PubMed: 32839655]
Previous CADTH Reports
Unclear Intervention and/or Comparator – Potential Intraoperative Opioid Use
Alternative Population – Intensive Care Unit
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for noncommercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Questions or requests for information about this report can be directed to Requests@CADTH.ca
- Research Question
- Context and Policy Issues
- Methods
- Summary of Evidence
- Limitations
- Conclusions and Implications for Decision- or Policy-Making
- Abbreviations
- References
- Selection of Included Studies
- Characteristics of Included Publications
- Critical Appraisal of Included Publications
- Main Study Findings
- References of Potential Interest
- Opioid-Sparing Effects of IV Acetaminophen for Patients Undergoing SurgeryOpioid-Sparing Effects of IV Acetaminophen for Patients Undergoing Surgery
Your browsing activity is empty.
Activity recording is turned off.
See more...
