This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
Chronic obstructive pulmonary disease (COPD) is a chronic progressive lung condition that results in substantial mortality, morbidity, and disability. Patients with COPD report unmet needs for information about their disease and guidance on how to manage and cope with it. Self-management support interventions can address these unmet needs. Among patients with COPD, self-management support interventions have resulted in improved health-related quality of life (HRQOL) while reducing acute-care use, yet it remains unclear how most effectively to provide self-management support to patients with COPD and their caregivers in health care settings. Peer support (ie, support provided by a person with a similar medical condition) has been used with positive results among patients with various chronic conditions, yet no randomized studies have focused on testing its effects for patients with COPD and their caregivers.
Objective:
The Better Respiratory Education and Treatment Help Empower 2 (BREATHE2) study aimed to compare the effect of self-management support strategies that incorporate health care professional (HCP) and peer support on HRQOL among patients with COPD.
Methods:
We used a 2-arm, single-blinded, randomized controlled trial design to compare the effectiveness of 2 strategies intended to engage patients and family caregivers in self-management of COPD. One strategy relied on the HCP as the primary communicator about COPD self-management (HCP Support); the other strategy used a dual approach that involved both HCPs and peer supporters to deliver tailored COPD self-management support (HCP Plus Peer Support). The HCP Support strategy included providing a written guide on COPD self-management, a 1-hour session with a respiratory care practitioner (RCP) at the start of the study period, and an RCP phone number to call with any follow-up questions or concerns over a 6-month intervention period. The HCP Plus Peer Support strategy included the same HCP Support strategy components plus an invitation for the participant to join a peer support program. The program provided peer support via 1-on-1 and group conversations with peer supporters over a 6-month intervention period. The peer supporters received training to provide peer support; they had COPD, had successfully stopped smoking, and had completed a pulmonary rehabilitation program, or they were caregivers for someone who had COPD. A peer support program coordinator was responsible for organizing and maintaining the peer program activities. Participants in the HCP Plus Peer Support arm were invited to attend 8 group gatherings in which self-management topics were discussed. The gatherings were co-led by the peer supporters and occurred over a 6-month period (1 meeting every 3 weeks). The peer supporters were also asked to reach out to each participant once they joined the program and, if the participant did not attend a gathering, to update him or her on the event discussions.
Our primary hypothesis was that patients in the HCP Plus Peer Support arm would have a greater improvement in HRQOL (as measured by the St. George's Respiratory Questionnaire [SGRQ] total score) at 6 months after discharge compared with baseline than would patients in the HCP Support arm.
The study was conducted at 1 academic and 1 community hospital within 1 health system and their affiliated clinics. The academic hospital served a low-income population living in an urban setting, while the community hospital was in a suburban area. The study population included patients aged ≥40 years who had been diagnosed with COPD by a physician and were currently receiving daily treatment for it. Each patient participant was invited to have 1 adult family-caregiver enroll with him or her in the study. Patients were recruited from inpatient and outpatient settings; all intervention activities occurred in outpatient settings only.
The primary study outcome measure was the change in HRQOL as measured by the SGRQ total score at 6 months compared with baseline. Secondary patient outcomes included the combined number of COPD-related and all-cause acute-care visits (hospitalizations and emergency department [ED] visits) per patient and patient-reported measures of self-efficacy, hope, and support. The measures were assessed at baseline and at 3, 6, and 9 months after enrollment.
Analysis of the treatment effect between the 2 study arms was conducted under intention to treat, and adjusted for baseline measures, site, and recruitment setting (inpatient vs outpatient). The primary hypothesis was evaluated with a mixed random effects model in which the main test of the hypothesis was of the interaction term of arm and baseline-to-6-month measurements on the SGRQ. Analyses were conducted unadjusted and adjusted for relevant patient baseline characteristics. Estimates of the intervention effect over time were evaluated, both unadjusted and adjusted for patient characteristics.
Results:
In total, 292 patient participants were randomly assigned to the 2 study arms. The majority of participants were White (70.9%) and female (61.3%). The mean age of participants was 67.7 (SD, 9.4) years. About half (51%) had an education level of some college or above, 41% had an annual income <$20 000, and 26.4% were on continuous home oxygen therapy. No significant differences in QOL at 6 months were found between the study arms. From baseline to 6 months, the mean change in total SGRQ score was −0.52 points in the HCP Plus Peer Support arm and −1.78 in the HCP Support arm (unadjusted difference of 1.26 points, with 95% CI, −5.44 to 7.96; P = .591). The participants randomly assigned to the HCP Plus Peer Support arm had fewer COPD-related acute-care events. After adjustment for baseline patient characteristics, the incidence rate ratio of COPD-related acute-care visits (hospitalizations and ED visits) between the HCP Plus Peer Support arm and the HCP Support arm was 0.68 (95% CI, 0.50-0.93; P = .016) at 3 months and 0.84 (95% CI, 0.71-0.99; P = .04) at 6 months. The participants randomly assigned to the HCP Plus Peer Support arm had significantly higher self-efficacy and hope scores than those in the HCP Support arm. Differences between the 2 study arms in acute-care visits, self-efficacy, and hope levels were no more significant at 9 months. Participation in peer support program activities was low, with an average number of peer encounters of 4.4 (SD, 4.2).
Conclusions:
We found no significant differences in QOL between the study arms; however, COPD-related acute-care use was significantly lower in the HCP Plus Peer Support arm during the 6-month intervention period, with significantly higher self-efficacy and hope levels.
Limitations:
The trial was conducted at 2 study sites within 1 health system. Treatment effects were different at the 2 study sites, with participants recruited at the suburban, more affluent site having better outcomes than those at the urban site. Of note, participation in program activities was also higher at the suburban site, with more participants having in-person interactions with their peers.
Assessment of treatment effects was limited by low participation rates in peer support program activities. More research is needed to examine how peer support interventions can be delivered in different settings and contexts and to assess their treatment effects.
Background
Chronic obstructive pulmonary disease (COPD) is a chronic progressive lung condition that results in substantial mortality, morbidity, and disability.1-3 COPD is the fourth leading cause of death in the United States and a leading cause of hospitalizations.3 Patients with COPD report unmet needs for information about their disease and guidance on how to manage and cope at “intellectual, emotional, and social levels.”4-6 Many patients with COPD lack the information and skills to correctly use inhaled medications,7,8 manage breathlessness episodes, and recognize early signs of COPD exacerbation.9-12
In a survey of 1102 people aged >40 years who have COPD in the United States, >77% reported an inability to perform daily activities, including exercising, climbing stairs, and walking.13 A concurrent survey of 1051 physicians revealed that 54% of primary care providers had inadequate knowledge about COPD practice guidelines and effective treatment options, including supplemental oxygen use and pulmonary rehabilitation.13 Deficits in general nurses' knowledge of COPD and proper inhaler use technique have also been reported.14,15 Recommendations to improve care for patients with COPD and reduce acute-care use include an increased focus on advancing patients' self-management skills (eg, proper use of inhalers and smoking cessation) and a call for initiatives that incorporate patients' and family caregivers' perspectives into the design of supportive, patient-centered programs to improve health outcomes.16,17 In a qualitative study of 34 participants with a range of quality of life (QOL) levels, the majority had significant challenges with coping and COPD self-management and asked for supportive services beyond pharmacologic interventions.18 Family caregivers of patients with COPD also report distress that affects their emotional well-being, professional life, and QOL overall.19-21 These caregivers, the majority of whom are spouses, frequently have their own life and health challenges.22 They commonly adopt a “1 day at a time” attitude to cope with the burden of caregiving,23 and their caregiving has been associated with improved patient outcomes.24
One approach to helping patients with COPD and their caregivers is to provide self-management support interventions in a manner that is sensitive to their needs and preferences. A systematic review of 29 COPD self-management studies concluded that COPD self-management education and support resulted in improved health-related QOL (HRQOL) and reduced hospitalizations and emergency department (ED) visits.25 Nonetheless, it remains unclear how most effectively to provide self-management support to patients with COPD and their caregivers in health care settings so that an improvement in patient outcomes can be realized.26,27
Medical guidelines for COPD management vary in regards to incorporating recommendations for provision of self-management support by health care professionals (HCPs).28-30 The Global Initiative for Chronic Obstructive Lung Disease recommends self-management interventions in communication with an HCP, given evidence of its impact on improving health status and reducing acute-care use.29 Self-management interventions aim to help patients improve their ability to manage COPD in their daily life and adopt desired health behaviors, including medication adherence, smoking cessation, adopting an active lifestyle, and participation in pulmonary rehabilitation.31 To help patients adopt these behaviors, coordinated follow-up and support from well-trained HCPs is needed, along with services to help patients overcome any barriers.17,31 Well-coordinated communication between providers is a challenging aspect of COPD care, as most patients with COPD have multiple comorbidities and see several providers.32,33 With the increased demand for pulmonologists' services in an aging population, most of the care for patients with COPD in the United States is provided by primary care clinicians.13,34 In 1 study, only 30% of patients with COPD reported that a pulmonologist was the main provider treating their respiratory problems.13 The majority of patients with COPD are treated in primary care clinics, where providers typically have less access to specialized respiratory care services or staff who are knowledgeable about inhaled treatments, supplemental oxygen devices, and the unique challenges that patients with COPD and their caregivers confront daily. The quality of health care and support services received by patients with COPD is variable and depends on treatment setting and available resources.17,35-37
Peer support (ie, support provided by a person with a similar medical condition) has been used to provide self-management support for patients with various medical conditions. Studies that use peer support for patients with obesity, mental health issues, addiction, diabetes, and cancer have shown increased self-efficacy and self-care behaviors as well as improved clinical outcomes and QOL.38-50 Examples of these programs include peer-led support groups, dyadic peer-to-peer mentorship, and online networks. Peer support benefits are attributed to the provision of emotional, informational, and appraisal support (ie, peer affirmation of the “appropriateness of one's emotions, cognitions, and behaviors”).51 Peer support benefits both those who provide it and those who receive it. Using peer support to improve self-management is particularly promising because the peer supporters (or peer mentors/persons providing support) have credibility as people “who understand, have been there, and done that.” Peer supporters can also model desirable health behaviors. These elements are key to achieving behavior change, according to the social learning theory.52 Peer support strengthens patients' social support while reducing isolation and may offer important benefits for patients who are hard to reach.53 Social support is particularly relevant to patients with COPD, whose medical illness often makes them reluctant to go out with family and friends because of functional limitations and the need for portable oxygen. Among patients with COPD, social support is associated with reduced hospitalizations, fewer exacerbations, and better health status.54-56
It is important to consider family members' involvement in self-management support interventions for patients with COPD. Family members play important roles as caregivers, and their support becomes increasingly important as patients become more dependent.19,23 Family caregivers may positively impact patients' health care behaviors. Among patients with COPD, those who have caregivers are less likely to smoke and more likely to adhere to medication regimens; they also use acute care less often.24,57 However, caregivers can also have a negative impact by being overprotective, which can lead patients to be more dependent and less active.20 Family-centered interventions to improve self-management among patients with chronic conditions have been reported to improve adoption of positive health care behaviors and reduce acute-care use.58,59
The Better Respiratory Education and Treatment Help Empower 2 (BREATHE2) study was a randomized controlled trial (RCT) that compared the effect of self-management support interventions that incorporate HCP and peer support on HRQOL and acute-care use for patients with COPD.60 Our overall goal was to compare the effectiveness of 2 health communication and dissemination strategies designed to engage patients and family caregivers in successfully managing COPD in “real-world” settings. Both strategies aimed to advance patient understanding of COPD, its treatment options, and self-care tasks; support participants in coping with the disease; and enable them to adopt a variety of positive behaviors (eg, adherence to treatment plans; smoking cessation; joining pulmonary rehabilitation programs; assuming an active, healthy lifestyle). One strategy relied on the HCP as the primary communicator about COPD self-management (HCP Support arm), whereas the other used a dual approach that involved both HCPs and peer mentors delivering such communication (HCP Plus Peer Support arm). Peer mentors were patients with COPD and caregivers who successfully managed COPD and had received foundational training on peer mentoring. Specifically, we aimed to (1) conduct an RCT in which the HCP Support and HCP Plus Peer Support strategies were tested in real-world health care settings; (2) compare the impact of these strategies on patient satisfaction, experience, activation, self-efficacy, self-care behavior, health status, QOL, use of ED and hospital services, and survival; and (3) compare the impact of these strategies on caregiver satisfaction, experience, self-efficacy, stress, and coping skills.
Patient and Stakeholder Engagement
In the BREATHE2 study, we used a participatory research approach in which we partnered with patients, caregivers, and stakeholders throughout all phases of the research. We started by composing a research team that included 1 patient and 1 caregiver as co-investigators who closely worked with the researcher co-investigators, attending research team meetings and making decisions as needed throughout the study period. The patient co-investigator has COPD and is an active member of the COPD Foundation and a strong advocate for patients with COPD. The caregiver co-investigator has been a caregiver for someone who had severe COPD for more than 30 years, and she served as a partner on 1 of our earlier studies.
To further ensure meaningful engagement of patients, caregivers, and stakeholders in this study, we closely worked with a patient and family partners (PFP) group that held its own meetings throughout the study period, as well as a group of health care and community stakeholders who were invited to joint study meetings held periodically, with all the PFP and the research team members invited. The PFP group was assembled at the start of an earlier PCORI-funded trial (the BREATHE study) and had an average of 12 members (patients with COPD and caregivers). Four of its original members have since passed away, and new members have joined. At the time of writing this report, the PFP group included 7 patients (4 women and 3 men) and 5 caregivers (3 women and 2 men). The PFP group weighed in on all research plans and helped develop study recruitment materials and study interventions.
The PFP group meetings were regularly attended by the study principal investigator (PI) and the patient and caregiver co-investigators. Agenda items were decided jointly with group members, discussions took place freely, and all members were encouraged to express their opinions. The idea for the BREATHE2 study originated from ongoing work over the past several years with the PFP group and various stakeholders, including physicians, social workers, respiratory therapists, homecare nurses, case managers, health care administrators, patient advocacy organizations, policy makers, and payers. During PFP meetings, the partners repeatedly voiced the need to connect with peers and receive information about COPD, its treatment, and ways to self-manage it. They proposed using groups “like this one” to communicate about these issues and support each other. All study recruitment materials were co-developed with the study partners and stakeholders. For example, the PFP group proposed creating a video to help with participant recruitment. The video described goals of the study and its interventions and provided first-person narratives related to COPD management. The partners proposed ideas about the key message for that video—namely, that “there is hope after COPD diagnosis and one may have good quality of life while living with COPD.” Patient and stakeholder perspectives also shaped the content of the informed consent document and the way we presented the study to potential participants.
Close work with the patient and caregiver co-investigators and the PFP group led to the interventions tested in this study. For example, the research team, including the patient and caregiver co-investigators, developed an initial set of opening questions for each group event and proposed icebreaker activities for the peer support program get-togethers. The initial plan was drafted as a table by the Intervention Development Workgroup, which included patient and caregiver co-investigators and researchers. This draft was then reviewed in detail at the study's joint team meeting, which included researchers, all PFPs, and stakeholders. We received further feedback from the PFP group on how to phrase the questions pertaining to patient-caregiver relationships and on specific icebreaker activities that the partners thought were worth using at multiple sessions. Based on this feedback, a final set of opening questions and icebreaker activities was developed.
The study partners and stakeholders also helped shape intervention design, choice of study outcomes, and planning for sustainability of study interventions. For example, the partners and stakeholders believed that QOL is an important outcome and supported its use as the primary outcome of this study. Toward the end of the study, the study partners and stakeholders were engaged in discussions about mechanisms for sustaining peer support delivery to interested study participants after the end of the research period. Together, we identified available peer support options in the areas where participants resided and informed participants about those options at the end of the study. These options included the COPD Foundation support line; Better Breathers clubs (sponsored by the American Lung Association); and a local support group facilitated by one of the BREATHE Pals, with support from 1 of the study sites (Howard County General Hospital [HCGH]). (See Table 2A in Appendix A for detailed examples of patients' and stakeholders' engagement impact on this study.)
More broadly, the elaborate, multipronged structure for patient and stakeholder engagement in this study—patient and caregiver co-investigators working with researchers; a separate partners group that met throughout the study period concurrent with research team meetings; and the joint meetings of partners, stakeholders, and researchers—affected all study team members by helping foster collegial relationships, open conversations, and iterative cycles of discussions and actions to address study challenges. The study structure allowed for relationship building among academic researchers, patient and family co-investigators and partners, and stakeholders; it also fostered empathy and understanding for the struggles of patients and caregivers who are striving to cope with COPD.
Methods
Study Overview
Helping patients follow treatment recommendations and adopt desired behavior is viewed as the responsibility of HCPs. Evidence-based guidelines for COPD management, provided by the American Thoracic Society and European Respiratory Society, include recommendations for smoking cessation, active lifestyle counseling, and education and self-management support.61 These guidelines are followed to a varying extent, depending on the care setting and the availability of health care team members who can implement them.
Reliance on HCPs to provide self-management support and promote behavior change, however, is limited by the lack of key features of behavior change theories, such as role modeling.52 Peer supporters (sometimes also called mentors) who share the same condition with patients can be effective and influential communicators. Studies that bring peers together and have peer supporters or mentors communicate about self-management have been successful in improving self-efficacy and clinical outcomes among patients with chronic diseases.38,40,62-65
The BREATHE2 study was a 2-arm, single-blinded RCT comparing the effectiveness of 2 strategies intended to engage patients and family caregivers in self-management of COPD in a real-world setting. One strategy relied on the HCP as the primary communicator about COPD self-management (HCP Support arm); the other strategy used a dual approach involving HCPs in conjunction with peer supporters to deliver tailored COPD self-management support (HCP Plus Peer Support arm). Both strategies aimed to advance patient and caregiver understanding of COPD, treatment options, and self-care and to encourage adoption of positive health behaviors, such as adherence to treatment plans, smoking cessation, participation in pulmonary rehabilitation programs, and active lifestyles.
The BREATHE2 study conceptual model, depicted in Figure 1, displays the mechanism by which HCPs and peers can help improve patient and caregiver outcomes. In the traditional medical expert model, HCPs are tasked with providing information about COPD, referring patients to treatment services and programs (eg, pulmonary rehabilitation, smoking-cessation programs), and supplying encouragement and support for patients to implement recommended self-care practices and desired behaviors (depicted in the blue arrow and box in Figure 1).
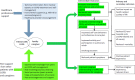
Figure 1
BREATHE2 Study Conceptual Model.
Although all these HCP contributions are essential to caring for patients with COPD, they may be insufficient to provide the needed support to induce and maintain positive behavior changes. According to social cognitive theory, self-efficacy mediates behavior change, and achieving self-efficacy requires the practice and mastery of necessary skills, modeling of desired behaviors, and social persuasion.52 Patients who have successfully learned to manage their COPD by adopting the necessary skills and desired behaviors (such as quitting smoking and completing a pulmonary rehabilitation program, both of which were requirements for being a peer supporter in this study) are consequently well positioned to help other patients with COPD (and their caregivers) by sharing personal experiences in managing COPD and the everyday challenges that it imposes. The ways in which peer support can help are depicted in the green arrow and box in the model. Peer supporters are uniquely able to provide other patients with COPD what HCPs cannot: the role modeling of desired behaviors and a hopeful and persuasive message that these behaviors are achievable.66 Peer support involves provision of relevant and credible information, delivered by positive role models who also experience reciprocal gains from this work in giving back and helping other patients who are experiencing a similar condition.51,67
A dual-support approach that involves pairing expert HCP services with peer support enables patients to receive (1) the technical information they need from HCPs and referrals to services that will enable them to adopt recommended behaviors; and (2) additional support from peers in the form of informational, instrumental, emotional, and social support as well as hopeful messages that increase the likelihood that they will become more activated and achieve the self-efficacy needed to successfully manage COPD. Although the self-management support that HCPs provide would be expected to increase self-efficacy and improve behaviors and outcomes,25,68-70 we expected the dual-support approach (HCP Plus Peer Support) would lead to more benefits than an approach that relied only on HCPs (HCP Support). Figure 1 depicts the intermediate outcomes where the dual-support approach is likely to exert its additional benefits on patient and caregiver outcomes (see the green highlighted text in model).
Study Hypotheses
The primary study hypothesis was that patients in the HCP Plus Peer Support arm would have a greater improvement in HRQOL (ie, a greater negative change in total score on the St. George's Respiratory Questionnaire [SGRQ]) at 6 months after discharge compared with baseline than would patients in the HCP Support arm (study aim 2). Our secondary hypotheses were that in a comparison with participants in the HCP Support arm, patient participants in the HCP Plus Peer Support arm (1) would have better HRQOL (ie, a greater negative change in total SGRQ score) at 9 months after enrollment and (2) would have reduced numbers of COPD-related acute-care visits (hospital and ED visits) at 3, 6, and 9 months. Additionally, we expected that these participants would have improved activation, self-efficacy, and self-care behaviors; a higher level of informational, instrumental, and emotional support; and less social isolation (study aim 2). We also expected that caregiver participants in the HCP Plus Peer Support arm would report a greater understanding of COPD and preparedness for caregiving than the caregivers in the HCP Support arm (study aim 3).
Study Design
A schematic of the study design is shown in Figure 2. The study used an RCT design with 2 arms. Patient participants were randomly assigned to either the HCP Support or HCP Plus Peer Support arm. Each patient participant was invited to have 1 adult family caregiver enroll with him or her in the same study arm, but this was not a requirement for study enrollment. The target for enrollment was 290 patients with COPD; we ultimately enrolled 292 patients. Fifty caregiver participants were also enrolled.
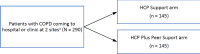
Figure 2
Schematic of Study Design.
All study participants received a written educational guide and a 1-hour session with a respiratory care practitioner (RCP), with the option to further contact the RCP by phone as they desired during the 6-month intervention period. Additionally, participants who had been randomly assigned to the HCP Plus Peer Support arm were invited to join a peer support program.
Patient and caregiver participants could not be blinded to their study arm assignment because of the design of the interventions, but the randomization assignment was concealed from all research team members performing data collection, medical records abstraction, and outcomes assessments. We also used standardized instruments to collect data and instructed the data collectors not to take notes that included patient information (to minimize recall in case of inadvertent disclosure of intervention arm assignment by a study participant to a data collector during data-collection interviews).
The study was approved by the Johns Hopkins Institutional Review Board, and written consent was obtained from all the study participants. The study is registered at ClinicalTrials.gov (NCT02891200: https://clinicaltrials.gov/ct2/show/NCT02891200?term=NCT02891200&draw=2&rank=1).
Study Setting
The study was conducted at 2 sites within the Johns Hopkins Health System: Johns Hopkins Bayview Medical Center (JHBMC) and HCGH, along with their affiliated pulmonary and primary care clinics. JHBMC is an academic medical center in Baltimore, Maryland, with 550 inpatient beds; it serves an urban population that has a large percentage of patients with low income. HCGH is a 300-bed community hospital in Columbia, Maryland, that serves a more affluent suburban population. Both sites have pulmonary specialty clinics on campus and operate large on-site pulmonary rehabilitation programs. The 2 sites also serve patients with pulmonary disease who receive care at primary care clinics that are part of the Johns Hopkins Community Physicians network.
Participants
Participant inclusion criteria were having a physician-provided diagnosis of COPD and receiving treatment for it (defined as receiving treatment at a hospital or clinic for COPD); being aged ≥40 years; having no non-COPD–related terminal illness (ie, life expectancy <6 months); and having no severe cognitive dysfunction, determined based on the patient's ability to follow instructions (ie, patient could provide informed consent). Exclusion criteria were inability to speak English; cognitive dysfunction impairing the patient's ability to provide informed consent and follow instructions; active substance abuse or unstable major psychiatric condition (as determined by the patient's health care team); a non-COPD–related terminal illness (ie, life expectancy <6 months); planning to move from the area; living in hospice care or long-term nursing home; or inability to provide contact information.
If patient participants had an adult family member (aged ≥18 years) involved in their health care, they were encouraged to invite that family member to enroll along with them in the study. Caregivers were excluded if they did not speak English or were unable to provide contact information.
Recruitment and Randomization
Participants were recruited from inpatient and outpatient settings. In the inpatient setting, a proactive approach was used to ensure that all patients with COPD who were admitted to the 2 study site hospitals were informed about the study. Materials about the study were distributed at the outpatient clinics, and providers were encouraged to refer their eligible patients to the study. For hospitalized patients, we used hospital patient censuses and diagnosis lists to identify potentially eligible candidates. These candidates were then approached by a study team member, who shared detailed information about the study and answered patients' questions. Candidates who were interested were consented and enrolled during their hospital stay and before their hospital discharge, whenever possible. The study start date for participants enrolled from the hospital was established as the date of their hospital discharge. Interested patients who could not be enrolled before discharge were subsequently contacted by phone to schedule an enrollment visit.
At the outpatient clinics, recruitment materials were available in waiting areas and distributed by health care providers to patients. Interested patients filled out an information card; a study team member then contacted them by phone to determine eligibility and schedule an enrollment visit. Study team members were also available at select outpatient sites to meet patients directly referred to the study by their health care provider. Later in the course of the study, patients with COPD who visited the pulmonary clinic at 1 of the study sites were mailed a letter informing them about the study.
If patient participants had an adult family member involved in their health care, they were encouraged to invite that family member to enroll with them in the study. If the participant approved, the family caregiver was approached for consent. Caregivers could be consented at the hospital, at the enrollment visit with the patient, or at a separate enrollment visit, depending on their availability.
After patient consent had been obtained, baseline data were then collected, a cognitive assessment and spirometry testing were conducted, and patients were randomly assigned in a 1:1 ratio to 1 of the study arms based on a pregenerated sequence of assignments. Randomization was stratified by 4 site-setting strata (JHBMC inpatient, JHBMC outpatient, HCGH inpatient, and HCGH outpatient), and a computer algorithm was then used to perform a blocked randomization assignment within strata, with randomly selected block sizes of 2, 4, or 6 participants. If a patient had a caregiver enrolled with them in the study, that caregiver was assigned to the same study arm as the patient.
Both patient and caregiver participants were compensated for their participation. Compensation was contingent on completion of a survey at each of the 4 data-collection time points (baseline, 3 months, 6 months, and 9 months). Patient participants were paid $20 at baseline, $15 at 3 months and 6 months, and $30 at 9 months. Caregiver participants were paid $15 at each time point.
Interventions and Comparators
The study had 2 arms: the HCP Support arm and the HCP Plus Peer Support arm. The HCP Support arm included providing patients and their caregiver a written guide on COPD self-management, a 1-hour session with an RCP at the start of the study period, and the RCP phone number to call as needed with any follow-up questions or concerns over the 6-month intervention period. The HCP Plus Peer Support arm included the same components as in the HCP Support arm, but in addition, the participants were invited to join the peer support program. This program provided peer support through 1-on-1 and group conversations with peer supporters over the 6-month intervention period. Below, we describe the HCP Support and HCP Plus Peer Support arms in more detail.
HCP Support arm
All participants in this study received HCP support from a trained RCP and a written guide on COPD self-management, which was co-developed with patients and family members as part of an earlier study and is described elsewhere.70 The RCP held a 1-hour individual session with each patient participant and his or her family caregiver, when possible. Sessions were held at the study site at which the participant usually received care. In these sessions, the RCP discussed COPD self-management, reviewed COPD medication use and inhaler technique, and discussed oxygen devices, as applicable. The sessions took place in person within 2 months of patient enrollment in the study. If patients missed their session, they were scheduled for another. If they missed the second appointment, the RCP reached out to them and offered to hold their session by phone. At the end of the session, the RCP provided a telephone number and email address to the participants, encouraging them to contact her with any questions or concerns during the 6-month study period.
HCP Plus Peer Support arm
Participants randomly assigned to the HCP Plus Peer Support arm received the same COPD self-management written guide, had the 1-hour session with the same RCP as in the HCP Support arm, and had the RCP phone number to call with questions over the 6-month intervention period. In addition, they were invited to join a peer support program for 6 months that was designed for patients with COPD. The program offered patient and caregiver participants peer support via multiple modalities, including 1-on-1 and group conversations held by phone and in person.
In the HCP Plus Peer Support study arm, participants were invited to meet other people who had COPD and their caregivers in a series of 8 group meetings, called get-together events, held every 3 weeks at the study site over a 6-month period. Participants were also matched with an individual peer supporter, called a BREATHE Pal. The peer support program coordinator, who was a licensed clinical social worker, provided program administration and organization. The peer support program coordinator also provided training, supervision, and support to the BREATHE Pals and matched them with the study participants. To the extent possible, matching of the BREATHE Pals and patient participants was conducted based on preset criteria: (1) study site (JHBMC or HCGH); 2) whether the participant was a patient or a caregiver (ie, patient participants were matched with a BREATHE Pal who had COPD; caregiver participants were matched with a BREATHE Pal who was a caregiver to someone with COPD); (3) use of oxygen therapy; and (4) sex. The BREATHE Pals could talk with the participants at the get-together events, by phone, or both, based on participant preference and attendance. As needed, the BREATHE Pals would communicate with the peer support program coordinator about any patients who were struggling with psychosocial issues or unmet needs; the peer support program coordinator would provide counseling to these participants and connect them with resources in the community.
Each get-together was co-led by2 BREATHE Pals and facilitated by the peer support program coordinator. Select predetermined topics about COPD self-management were discussed at each meeting, and the messages delivered by the RCP were reinforced (See Table 3A in Appendix A for a detailed list of discussion topics).
Peer Support Delivery
Following their match to a new participant, assigned BREATHE Pals would call the participants they were matched with to introduce themselves, discuss their role, and share commonalities in their experiences with COPD. During this initial call, the BREATHE Pal ascertained whether the patient had any unique needs for participating in the program, such as mobility limitations, dietary requirements, or needing assistance with transportation to attend get-togethers. If patients were using oxygen, they were advised to bring a full oxygen tank to get-togethers. Before each get-together, BREATHE Pals would call to confirm participants' attendance and whether a family caregiver would be joining them.
Peer support group conversations occurred over a 6-month period at 8 get-together events, and each event had a specific theme. If participants missed a get-together for any reason (eg, personal preference or health issue), they could call in to a similarly themed monthly group teleconference call to catch up on missed topic conversations. Additionally, their BREATHE Pal called them afterwards to check in, update them on conversations that took place at any get-together that they had missed, answer any questions, and encourage them to attend the next event. These ongoing communications helped the BREATHE Pals develop individual relationships with each person with whom they were matched. The peer support program coordinator carefully monitored each BREATHE Pal's level of comfort with program activities and the number of matched participants so that no BREATHE Pals were burdened by their role.
At each group event, the participants and BREATHE Pals would discuss COPD and share their experiences in coping with and managing it. The get-togethers followed a rotating sequence of 8 themes. Because new participants were not started in cohorts but began their participation from the date of their enrollment, having a rolling schedule granted all participants the opportunity to participate in the full breadth of the program; participants could join the event sequence at any time and continue for 8 get-togethers, thus participating in all 8 discussions. If at any point the number of participants attending get-togethers at a site exceeded 20 people, a new group was initiated. (Table 3A in Appendix A shows the get-together themes and topics in detail.)
The get-togethers provided an open forum for patients and their caregivers to share their experiences with COPD and managing its various impacts on their lives. Each get-together lasted 90 minutes. The BREATHE Pals used a set of suggested icebreaker activities and opening questions to start the sessions. Examples of openers included sharing a personal story related to the meeting topic or sharing coping strategies, such as pursed-lip breathing, to start the discussion. Participants were also encouraged to discuss any COPD-related issues that were affecting them presently. Lunch was provided at the meetings, and time was reserved for participants to socialize at the end of each meeting (approximately 30 minutes). This was an informal way for participants to network and bond. Any individual participant concerns could be discussed at that time between the participant and his or her BREATHE Pal and/or the peer support program coordinator.
Peer Supporters' (BREATHE Pals') Identification, Role, and Training
BREATHE Pals were required to have COPD and not be current smokers; they also had to have completed an acute pulmonary rehabilitation program. Family caregivers of individuals who met the BREATHE Pal criteria were also eligible to become BREATHE Pals as a caregiver peer supporter.
Prospective BREATHE Pals were initially nominated by a pulmonologist or a pulmonary rehabilitation staff member at 1 of the study sites. We reached out to the providers and teams at the clinics and rehabilitation centers to ask for nominations of individuals who met the eligibility criteria and had the appropriate skills and disposition for peer mentorship. Later in the study, candidates were also found among HCP Plus Peer Support participants who had completed their participation in the study.
Candidates were then interviewed by the peer support program coordinator about their ability and motivations to become a peer supporter. Candidates then received orientation and training on how to provide peer support over the course of four 3-hour training sessions. Examples of training topics included engaged/active listening, effective communication, building trust and partnership, answering common questions and concerns for people with COPD, recognizing red flags and crises, and learning how to share their personal stories and experiences. The teaching techniques employed included group discussion, storytelling, and role-playing. Candidates who completed training and were still interested in becoming BREATHE Pals were formally consented. BREATHE Pals were validated as formal volunteers at the respective study site hospitals after completing institutional requirements (eg, background checks, privacy and emergency preparedness training).
During the program, the BREATHE Pals received ongoing support and guidance from the peer support program coordinator in person and by phone. The peer support program coordinator ensured that the BREATHE Pals followed program procedures and policies, which included not giving medical advice and remaining nonjudgmental. She also helped facilitate difficult conversations within the group at the get-togethers, though the BREATHE Pals led the discussions.
Upon enrollment, each BREATHE Pal committed to serve in that role for at least a 9-month period to maintain continuity between participants and matched BREATHE Pals. At a minimum, the BREATHE Pals were expected to call the participant initially, and then after any get-togethers that the participant missed.
Study Outcomes
In this study, we measured primary and secondary patient outcomes, intermediate outcomes (see Figure 1), and caregiver outcomes. We also assessed implementation of intervention activities in both study arms and patient experiences with the peer support program.
Primary Outcome (Patient)
The primary study outcome measure was change in HRQOL as measured by the SGRQ total score at 6 months after enrollment compared with baseline. The SGRQ is a validated, standardized, self-reported instrument for measuring HRQOL among people with respiratory conditions. It consists of 76 items divided into 3 domains: Symptoms, Activity, and Impacts. A total score is calculated to determine the impact of disease on overall health status. All scores (total and domain scores) are on a scale from 0 to 100, with higher scores indicating worse QOL. The threshold for clinical significance (minimally important clinical difference) on the SGRQ is a difference of 4 points.71 Patients who died were given a score of 100 (worst possible score).
Secondary Outcomes (Patient)
The study included the following secondary outcomes:
- The change in HRQOL from baseline to 9 months, as measured by the SGRQ total score, as well as the changes in the SGRQ domain scores. (See details on the SGRQ under “Primary Outcome [Patient]”.)
- COPD-related and all-cause acute-care use, measured as (1) the combined number of COPD-related acute-care visits (hospitalizations and ED visits) from enrollment up to each study time point (3 months, 6 months, and 9 months) for all participants who were alive and still enrolled in the study at that time point; and (2) the combined number of all-cause acute-care visits (hospitalizations and ED visits) from enrollment up to each study time point (3 months, 6 months, and 9 months) for all participants who were alive and still enrolled in the study at that time point.
- The data on hospital and ED visits included all visits within the Johns Hopkins Health System as well as hospitals within Maryland, Delaware, and the District of Columbia. Data were obtained on all study participants from enrollment up to the 9-month time point. (See the “Data Collection and Sources” section for more details.) We determined whether a visit was COPD related by using a computer algorithm based on a set of predetermined discharge diagnoses that indicate COPD-related reasons (principal ICD-10 diagnosis [Dx] of J41.0, J41.1, J41.8, J42, J43.0, J43.1, J43.2, J43.8, J43.9, J44.0, J44.1, J44.9; or principal Dx of J96.00, J96.01, J96.02, J96.21, J96.22, J96.90, J96.91, J96.92, R06.03, R09.2 WITH a secondary Dx of J44.0 or J44.1).
- Mortality was assessed by measuring the mortality rate. We also conducted a “time to event” survival analysis, with the combined event of death or first COPD-related acute-care event (hospitalization or ED visit—whichever occurred first) as the end point.
Intermediate Outcomes (Patient)
The intermediate outcomes assessed in this study were those on the pathway to improving health outcomes that we expected the tested interventions to change. (See Figure 1). Those outcomes included patient perceptions of support,72,73 patient activation,74 hope,75 self-efficacy and self-care behaviors,76 smoking cessation, and participation in pulmonary rehabilitation. These outcomes were measured via patient self-report at baseline, 6 months, and 9 months after enrollment.
The study included the following intermediate patient outcomes:
- Patient perceptions of support were measured using the PROMIS® measures of Emotional Support (perceptions of being cared for and valued by others), Informational Support (perceived availability of information/advice), Instrumental Support (perceived availability of assistance with material, cognitive, and task performance), and Social Isolation (perceptions of exclusion or disconnection from others).72,73 Measurements for each domain consist of T-scores, which are standardized scores with a mean of 50 and an SD of 10; higher T-scores represent a higher level of the measured concept. Higher T-scores for Emotional Support, Informational Support, and Instrumental Support reflect increased levels of support. Higher T-scores for Social Isolation reflect increased levels of social isolation and decreased support.
- Patient activation was measured using the Patient Activation Measure (PAM).74 PAM scores range from 0 to 100, with higher scores indicating more activation.
- Patient levels of hope were measured by the Herth Hope Index (HHI),75 which has total scores ranging from 12 to 48; higher scores indicate greater levels of hope.
- Self-efficacy and self-care behaviors were measured using an adapted version of the Understanding COPD (UCOPD) questionnaire.76 The UCOPD questionnaire measures patient report on understanding of COPD and patient confidence and use of COPD self-management skills. Questions were selected from 3 domains: About COPD, Managing Symptoms, and Accessing Help and Support. We followed a similar approach to calculating composite overall and domain scores in our study to that published in the UCOPD manual instructions. Scores are expressed as percentages of the maximum possible score, with 100% reflecting the highest understanding and self-efficacy and 0 the lowest.
- Smoking status was assessed at baseline and each follow-up time point by asking the question, “Are you currently a smoker?” with the following response options: “Yes, I am currently a smoker” or “No, I am currently not a smoker.”
- Pulmonary rehabilitation participation was assessed via a 2-part self-report question: “Have you participated in a pulmonary rehabilitation program?” If the patient responded “Yes,” he or she had to choose 1 of these responses: “I currently am,” “I have participated in it in the past 2 years,” or “I did participate in it more than 2 years ago.”
Caregiver Outcomes
Family-caregiver outcomes included preparedness for caregiving,78 caregiver stress,79 caregiver coping skills,80,81 and caregiver perceptions of support.72,73 All were assessed by self-report:
- Preparedness for caregiving was assessed using the Preparedness for Caregiving Scale (PCS), an 8-item instrument that asks about self-perceived readiness for different aspects of the caregiving role. Higher scores indicate more preparedness.78
- Caregiver stress level was measured using the Zarit Burden Interview. Stress index scores range from 0 to 16, with higher numbers indicating higher caregiver stress level.79
- Caregiver coping skills were measured by the Seeking Social Support Scale of the Ways of Coping Questionnaire, which measures caregivers' help-seeking behavior.80,81 The scale ranges from 0 to 18, with a higher score meaning that a caregiver used more coping behaviors that involved seeking social support.
- Caregiver understanding of COPD, measured using the total score on an adapted version of the UCOPD questionnaire76 that is tailored for caregivers. We followed a similar approach to calculating overall scores to that published in the UCOPD manual instructions. Higher scores on UCOPD are better.
- Caregiver experience was measured by assessing caregivers' perceptions of emotional and informational support. We used the PROMIS measures of Emotional Support (perceptions of being cared for and valued by others) and Informational Support (perceived availability of information/advice).72,73 Measurements for each domain consist of T-scores, which are standardized scores with a mean of 50 and an SD of 10; higher T-scores represent a higher level of the measured concept. Higher T-scores reflect increased levels of support.
Patient Experiences With the Peer Support Program
Participant experiences with the peer support program were collected via a phone survey at the end of the study period. The survey included closed and open-ended questions. Survey questions, including ratings of intervention activities, report on areas that participants felt most helpful, as well as recommendations for further improvement.
Intervention Implementation
We systematically tracked the number of times participants had sessions with the RCP; attended get-togethers; or had phone calls with a BREATHE Pal using forms that the RCP, peer support program coordinator, and the BREATHE Pals filled out after each encounter. Calls with the RCP and peer support program coordinator were similarly tracked and their content documented. (For more information about patient and caregiver outcomes and covariates, please see Table 4A in Appendix A.)
Sample Size Calculations and Power
The sample size calculation was based on an overall comparison of the change between the baseline and 6-month measurements of patients on the SGRQ (primary outcome) in the 2 arms (interaction term). The unadjusted per-arm sample size was based on a power of 0.80, an α of .05, a minimum clinically significant SGRQ score change of 4 points, and an estimate of variability taken from a meta-analysis.71 The estimated sample size was 145 patients per arm after accounting for a 15% attrition rate, assuming a within-patient correlation between measurements of 0.8.
Data Collection and Sources
We collected data on patient demographics, disease severity, comorbid conditions,82 self-ratings of physical and emotional health, health literacy,83 health care use, and anxiety and depressive symptoms. For caregiver participants, we collected data on age, sex, employment, relationship to patient, caregiving responsibilities, health status, and means of transportation.
Data were collected in person at baseline. At 3 months, 6 months, and 9 months, data were collected via phone interview by a research team member who was blinded to the participant's study arm allocation. No 9-month follow-up calls were conducted for participants who were enrolled after September 2017 because of the scheduled end-of-study period in June 2018. Up to 6 attempts were made to reach participants at each follow-up data-collection period. Detailed information about study variables and the data-collection methods is provided in Table 4A in Appendix A.
Information was also collected about all patient visits to the ED or hospital from 9 months before enrollment and up to 9 months after enrollment, along with the reasons for these visits. Based on our earlier studies and the medical literature, we determined that patient self-report of hospital and ED use is highly inaccurate as a measure of acute-care use because of recall bias. Furthermore, about 1 in 5 acute-care visits may occur outside the Johns Hopkins Health System where study participants are receiving their routine treatment services for COPD and are therefore inaccessible via review of participant medical records at the study sites. We therefore sought and obtained approval to obtain data on participants' ED and hospital visits from the Maryland Health Services Cost Review Commission, with the assistance of the Chesapeake Regional Information System for our Patients (CRISP).84,85
By legislative mandate (Code of Maryland Regulations [COMAR] 10.37.06 and COMAR 10.37.04), all acute-care hospitals in Maryland are required to submit confidential patient-level data on all acute-care hospital and ED visits, with principal and secondary diagnostic and procedure codes, discharge status of the patient, and types of services rendered. This requirement enabled us to obtain complete data on all acute-care visits of study participants within the state of Maryland. Data on participant deaths (for those we were unable to reach) was confirmed via the Maryland Department of Health Vital Statistics Administration.
To assess participant experiences with the peer support program, we conducted a phone survey at the end of the study to elicit qualitative feedback from all patient participants randomly assigned to the HCP Plus Peer Support arm. We called all patient participants at least once and left a message for them to call back if no one answered the phone.
Time Frame for the Study
All patient participants were interviewed in person by a trained study team member upon enrollment, before randomization. Subsequently, they were interviewed by phone at 3, 6, and 9 months after enrollment by a research team member who was blinded to study arm assignments. Caregiver participants were interviewed upon enrollment and subsequently at 3, 6, and 9 months after enrollment. Patient and caregiver participation in get-together events as well as the number of calls with the BREATHE Pals and the topics discussed were tracked.
Handling of Missing Data
There are 2 types of missing data: at the respondent level (unit nonresponse) and at the specific question level (item nonresponse). At the respondent level, missing data occurred because of patient death, withdrawal from the study, or inability to be reached by phone for an interview. For the SGRQ measure, values of 100 (the worst possible HRQOL score) were imputed for patients whose data were missing because of death. No other imputations were made for missing values at the respondent level. Generalized mixed random effects (RE) models were used for statistical analysis, which allowed for including repeated measures data for all the other time points when the participant did respond.
For missing data at the specific question level (item nonresponse), missing data were handled according to each outcome measurement instrument's manual and authors' directions. When no guidance was available on a particular instrument, a rule allowing no more than 25% of the items for the scale/domain to be missing was used. If <25% of the item responses were missing, the mean of the nonmissing item responses was calculated and used to replace the value of the missing item responses before calculation of the scale/domain score. If >25% of the item responses were missing, the scale/domain score was considered missing.
Analytical and Statistical Approaches
Statistical summaries and distributions of patient characteristics were reviewed by study site and setting within and across study arms. Based on the randomization of patients, we expected the study arms to be reasonably balanced on characteristics that might affect the study outcomes. Exploratory analyses were performed cross-sectionally at baseline, 3, 6, and 9 months after discharge for applicable outcomes across the 2 study arms. This approach provided an assessment of the outcomes' distributions and missingness patterns.
The main analysis approach for assessing the change in the primary outcome measure from baseline, as well as changes in secondary outcome measures from baseline, consisted of analyses of the treatment effect between the 2 study arms under intention to treat (ITT), adjusted for baseline measure, site, and recruitment setting (inpatient vs outpatient).
For all hypotheses, the main independent variable was the treatment arm assignment. The primary hypothesis was evaluated with a mixed RE model, where the main test of the hypothesis was of the interaction term of arm and baseline to 6-month measurements on the SGRQ. The mixed RE model reflects the study's interest in comparisons of change at the individual level, where the changes may be subject specific and reflective of potentially unmeasured variables. This model also fits well with the study's approach to missing data and heterogeneity of treatment effect (HTE) and will help us handle the anticipated missing 9-month data for participants who enrolled in the final period of the study. Analyses were conducted unadjusted and adjusted for patient characteristics such as age and sex. Estimates of the intervention effect over time were evaluated, both unadjusted and adjusted for patient characteristics.
For secondary outcomes, we fitted appropriate generalized linear mixed RE models based on the type of outcome variable. Continuous measures were assessed using linear mixed RE models and binary outcomes using logistic mixed RE models. Differences in change in outcome from baseline to 3, 6, and 9 months between the study arms were assessed by including interaction terms between the time variables and the study arm. We assessed significance by first testing the overall interaction between time and arm. If the overall interaction was significant for an outcome measure, we then estimated and tested individual time differences, accounting for multiple testing using a Bonferroni correction. Initially, models were adjusted only for baseline measure, site, and recruitment setting. We then adjusted for relevant patient demographic and clinical characteristics, such as age, sex, smoking, home oxygen use, and prior hospitalization. For acute-care use, such as number of hospitalizations and ED visits, we fit negative binomial models for each time period. These models were also initially adjusted only for baseline measure, site, and recruitment setting, and then additionally adjusted for patients' characteristics.
We performed unadjusted survival analyses using Kaplan-Meier and log-rank tests for time to death or first COPD-related hospitalization or ED visit. Statistical significance was considered for P < .05.
Given the nature of the study interventions, we anticipated that not all participants could fully participate in peer support program activities. To assess adherence, we measured the number of sessions held with the RCP and, for participants in the peer support program, the number of get-together events attended and the number of times they had a phone interaction with their BREATHE Pal. All conversations between study participants and the peer support program coordinator were also documented. We defined participants as adherent if they had ≥4 encounters with their peers or BREATHE Pal, either by attending a group event or having a phone conversation with their BREATHE Pal.
We conducted HTE analyses to explore the difference in treatment effect between the arms in subgroups of patients with characteristics that might affect the primary outcome of change in total SGRQ score from baseline to 6 months after enrollment. We conducted HTE analyses for the subgroups defined by the variables hospital site (HCGH vs JHBMC), enrollment setting (inpatient vs outpatient), sex, baseline activation level (low: PAM levels 1, 2 vs high: PAM levels 3, 4), age (<55 years vs 55-64 years vs 65-74 years vs ≥75 years), oxygen use, past hospitalizations, living alone, diagnosis of congestive heart failure (CHF), and similarity to compliers (low vs high, based on the similarity of baseline patient characteristics to those in the HCP Plus Peer Support arm who met our prespecified adherence definition of having at least 4 peer encounters either by attending a group event or having a phone conversation with their BREATHE Pal). These subgroup effects were estimated by including a term for the 3-way interaction between the subgroup variable, the study arm variable, and the 6-month time variable within the linear mixed RE model. We tested for differences in treatment effect between subgroups by using a hypothesis test of the overall 3-way interaction in this model. We also estimated the subgroup treatment effects by using linear combinations of coefficients from this interaction model.
Qualitative data from the participant experience survey, as well as RCP and peer support program coordinator notes on calls with participants, were analyzed using a thematic analysis approach by team members who have clinical training and expertise with qualitative data analysis.
Changes to the Original Study Protocol
In the original study protocol, we included spirometric criteria for enrollment in the study in addition to a physician diagnosis and receiving daily treatment for COPD. We had to remove this criterion in the first few weeks of the study, however, given the low percentage of patients hospitalized with acute exacerbations who had prior spirometry results available (<20%). Spirometry testing also led to ambiguous results among patients with established COPD and concomitant cardiopulmonary diseases (a common comorbidity), which could lead to their exclusion. Requiring spirometry testing as a condition for enrollment would have led to exclusion of a large number of patients, thus reducing study generalizability and, potentially, power. This criterion was also not consistent with the real-world approach we were following in this trial.
In the study planning phase, we decided with PCORI and IRB approval to change sample size from the initially proposed 325 participants to 290 participants. During our work on intervention development, we considered approaches to maximize patient engagement and decided to minimize patient waiting time to start the intervention. This decision required us to change from our initially proposed closed-group design to an open-group format where participants could join an already-initiated group as soon as possible after enrollment in the study. We reorganized peer group discussions and activities to engage new study participants as they joined existing groups and developed a rotating meeting agenda where a series of topics (1-8) would repeat, thus allowing new patients to join at any time for 8 sessions and still have the opportunity to discuss all topics. With this approach, we did not need to adjust for intraclass correlation within closed groups, which had originally inflated our target sample size. This change in group format and sample size did not affect study design, anticipated intervention effects, or the statistical power to show differences in outcomes.
Smoking history of >10 pack-years was initially included as a criterion for enrollment in the study. We later obtained approval (as of July 2018) to remove this criterion in an effort to streamline recruitment for the study. We opted initially to use this criterion so as to be consistent with other COPD trials, which used it to provide additional confirmation for COPD diagnosis. However, studies show that up to 20% of patients with COPD have no personal history of smoking (and indeed, this evidence roughly matched the percentage we had to exclude from the trial because of lack of smoking history). For those patients, passive smoking and environmental pollutants are commonly implicated. Several potential study candidates at both sites were nonsmokers and had expressed interest in joining in the study, and the PFP group thought it was not fair to deny them that option. Given this situation and our interest in expediting recruitment, we moved forward on the decision to remove the smoking history requirement.
Partway through the recruitment period, because of low recruitment at one of the study sites (HCGH), we obtained IRB approval for a change in recruitment protocol, allowing us to send a joint letter from the medical director of the HCGH pulmonary clinic and study PI introducing the study to those patients who had visited the clinic over the past year. The mailing also included informational materials about the study and how to participate in it.
Results
In our initial research proposal, we asked the following research question: Among patients with COPD and their caregivers, would a dual strategy that combines HCP and peer mentor delivery of COPD self-management education and support (HCP Plus Peer Support) result in greater improvements in health status and QOL as well as reductions in use of acute health care services compared with relying on HCPs alone (HCP Support) in these communications? Would such a dual strategy result in reduced caregiver stress and improved coping and satisfaction?
Research Question 1
Would a dual strategy that combines HCP and peer mentor delivery of COPD self-management education and support (HCP Plus Peer Support) result in greater improvements in health status and QOL as well as reductions in use of acute health care services?
Overview of Patient Participant Flow and Baseline Characteristics
Figure 3 depicts patient participant flow. From April 2017 to December 2018, we screened 1464 patients at JHBMC, HCGH, and their affiliated clinics, of whom 1061 patients were eligible for enrollment in the study. Among eligible patients, 434 declined to participate, and we were unable to contact 335 patients for enrollment. Reasons for declining to participate included lack of time or interest, concomitant medical problems, and family issues. Reasons for excluding participants included no COPD diagnosis by a physician or not receiving daily treatment for COPD (n = 79), severe cognitive dysfunction (n = 65), smoking history <10 pack-years (n = 50; this was an exclusion criterion at study start and was removed in July 2018 to expedite the recruitment timeline), active substance use (n = 45), living in a facility (n = 39), and unstable major psychiatric condition (n = 26). Nine patients were excluded because of homelessness, and 22 patients were excluded because they did not understand English. (See Table 5A and Table 6A in Appendix A for more details on the demographic characteristics of eligible, enrolled, and declined patients and reasons for eligible patients declining to participate.)

Figure 3
Patient Participant CONSORT Diagram.
Table 1 summarizes the baseline characteristics of enrolled participants by study arm. Most patient characteristics were similar in the 2 study arms. The majority of patients were White (70.9%) and female (61.3%). About half (51%) had an education level of some college or above. In total, 41% had an annual income <$20 000. At baseline, 26.4% of patients were on continuous home oxygen therapy, and 24.7% had participated in pulmonary rehabilitation. In total, 60% of patients reported a Modified Medical Research Council (mMRC) Dyspnea Scale breathlessness grade of 3 or 4, meaning that they stop for breath after walking short distances, are too breathless to leave the house, or are breathless while dressing. About 30% of participants were living alone, and 25% were currently smoking. There was a small difference in the percentage of smokers at baseline between the 2 study arms (27.9% in the HCP Plus Peer Support arm compared with 21.4% in the HCP Support arm) and a large difference in the percentage of patients with a diagnosis of CHF (42.9% in the HCP Plus Peer Support arm compared with 26.9% in the HCP Support arm).
Table 1
Patient Baseline Characteristics.
The baseline characteristics of patient participants who completed the study's 6-month follow-up surveys were compared with those who did not complete them. There were statistically significant differences between those observed and not observed at 6 months in terms of continuous oxygen treatment (21.8% among those observed, 40.3% among those missing; P = .002) and in terms of current smokers (20.9% among those observed, 36.1% among those missing; P = .009). We found similar differences for these 2 variables in each study arm (17.8% vs 45.0% on continuous oxygen among those observed vs missing in the HCP Plus Peer Support arm and 25.7% vs 34.4% in the HCP Support arm; 24.3% vs 37.5% smokers among those observed vs missing in the HCP Plus Peer Support arm and 17.7% vs 34.4% in the HCP Support arm). Because our questions of interest focused on differences in the change over time between the 2 treatment arms, it is likely that this missingness would affect the 2 treatment arms in a similar way. No other significant differences were found. (See Table 7A in Appendix A for patient characteristics by missingness at 6 months for the primary outcome.)
Impact on HRQOL
Our primary outcome in this study was change in HRQOL, as measured using the SGRQ total score, from baseline to 6 months after enrollment.
Table 2 compares the baseline scores and unadjusted raw differences from baseline to 6 months after enrollment for a total SGRQ score between the study arms among participants who had data at both time points. There were 113 and 107 participants with SGRQ data at both baseline and 6 months in the HCP Support and HCP Plus Peer Support arms, respectively. The participants with missing SGRQ data at 6 months were significantly more likely to be smokers (36.1% compared with 20.9%; P = .009) and to be on continuous oxygen (40.3% compared with 21.8%; P = .002) but were otherwise not significantly different in terms of baseline characteristics from the rest of the study participants. From baseline to 6 months, the mean change in total SGRQ score was −0.52 points in the HCP Plus Peer Support arm and −1.78 in the HCP Support arm (unadjusted difference of 1.26 points, with 95% CI, −5.44 to 7.96; P = .591).
Table 2
Unadjusted Change in HRQOL as Measured by Total SGRQ Score From Baseline to 6 Months After Enrollment.
Adjusted differences for mean change in total SGRQ score from baseline to 6 months were estimated using linear mixed-effects models that considered SGRQ scores at all 3 time points (baseline, 6 months, and 9 months) to take advantage of all available SGRQ patient data. Table 3 shows the adjusted differences between the treatment arms. After adjustment for baseline SGRQ domain scores, hospital site, and enrollment setting, there was no significant difference between the treatment arms (adjusted difference of 1.46 points, with 95% CI, −2.47 to 5.38; P = .467). This remained true with additional adjustment for a set of baseline patient characteristics (adjusted difference of 1.82 points, with 95% CI, −1.76 to 5.40; P = .319).
Table 3
Mean Change in HRQOL, as Measured by SGRQ Total Score From Baseline to 6 and 9 Months After Enrollment.
As a secondary outcome, we considered the change in SGRQ total score at 9 months after enrollment; adjusted differences between the study arms for this outcome are included in Table 3. We saw similar results to what was observed at the 6-month time point: no statistically significant differences in the mean change in total SGRQ score between the study arms when adjusted for baseline score, hospital site, and enrollment setting (P = .404) or when further adjusted for additional baseline patient characteristics (P = .219). Table 8A in Appendix A shows the SGRQ domain scores by study arm.
Impact on Use of ED and Hospital Services
As secondary outcomes, we assessed the study interventions' impact on use of ED and hospital services. Data on use of these services were available for all participants who enrolled in this study. Table 4 shows the mean number of COPD-related acute-care visits per participant at 1, 3, 6, and 9 months for the study arms. Patients who had died or withdrawn from the study were excluded from analysis starting from the time in which they died or decided to withdraw from the study. At 6 months, the mean number of visits was 0.62 in the HCP Plus Peer Support arm compared with 0.79 in the HCP Support arm (absolute difference, −0.17 visits [95% CI, −0.62 to 0.28; P = .312]).
Table 4
COPD-Related Hospitalizations and ED Visits Over the 6 Months After Enrollment, by Study Arm.
Table 5 shows COPD-related and all-cause event rates by study arm and incidence rate ratios (IRRs) comparing the study arms at 1, 3, 6, and 9 months after enrollment. Patients who had died or withdrawn from the study were excluded from analysis starting from the time in which they died or decided to withdraw from the study. IRRs were calculated after adjustment for hospital site and enrollment setting and after additional adjustment for baseline patient characteristics. After adjustment for baseline patient characteristics, the IRR of COPD-related visits at 1 month, comparing the HCP Plus Peer Support arm with the HCP Support arm, was 0.46 (95% CI, 0.30-0.70; P < .001). The IRR of COPD-related visits at 3 months, comparing the HCP Plus Peer Support arm with the HCP Support arm, was 0.68 (95% CI, 0.50-0.93; P = .016). The adjusted IRR of COPD-related visits at 6 months was 0.83 (95% CI, 0.70-0.98; P = .028). There was no significant difference in the rate of COPD-related visits between the study arms at 9 months. There was a significant difference in the rates of all-cause visits at the 1-month time point between the study arms (IRR, 0.65; 95% CI, 0.51-0.83; P = .001). There were no significant differences in all-cause visits at the 3-, 6-, or 9-month time points.
Table 5
IRRs of COPD-Related and All-Cause Acute-Care Visits Across the Study Arms at 1, 3, 6, and 9 Months.
Figure 4 shows the Kaplan-Meier survival curves for the time-to-event survival analysis, with the combined event of death or first COPD-related acute-care event (hospitalization or ED visit, whichever occurred first). The separation in survival curves is mostly seen in the 30- to 60-day period after enrollment; however, this difference was not statistically significant (log rank test; P = .834).
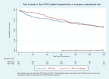
Figure 4
Time to First COPD-Related Acute-Care Event or Death.
Impact on Mortality
There were no significant differences in mortality rates between the study arms at any of the 3 study time points (Table 6). At 3 months, there were 5 (3.5%) deaths in each arm. At 6 months, there were 9 (6.3%) deaths in the HCP Plus Peer Support arm compared with 10 (6.9%) in the HCP Support arm. At 9 months, there were 13 (9.1%) deaths in the HCP Plus Peer Support arm compared with 15 (10.4%) in the HCP Support arm.
Table 6
Mortality Rate.
Research Question 2
Would the dual strategy (HCP Plus Peer Support) result in reduced caregiver stress and improved coping and satisfaction?
Caregiver Participant Flow and Baseline Characteristics
Figure 5 shows caregiver participant flow. Among 292 enrolled patient participants, 155 declined to have a caregiver enrolled, and 45 reported they did not have a caregiver. Among the remaining 92 patients who were interested in having a caregiver enrolled, 37 named a caregiver who was not afterwards available to enroll, and 5 named a caregiver who later declined to enroll. Fifty caregivers were enrolled in the same arm as the respective patient (21 in the HCP Support arm; 29 in the HCP Plus Peer Support arm). More caregivers enrolled and more were lost to follow-up in the HCP Plus Peer Support arm, likely because we allowed caregivers to continue to enroll after randomization of the patient participant (as many were not available because of work commitments to be present at the patient enrollment visit). Although more caregivers were interested in enrolling when they knew that their loved one had been randomly assigned to the HCP Plus Peer Support arm (as they were invited to participate in all intervention activities), they were less interested in participating in study assessments.

Figure 5
Caregiver CONSORT Diagram.
Table 7 shows the baseline characteristics of the caregivers enrolled (n = 50) by study arm. Average age of the caregivers was 60 years, and 66% were women. More than half of the caregivers were spouses or partners (58%). In total, 28% of the caregivers were employed, and 32% were current smokers. Among enrolled caregivers, 72% reported having their own health challenges, and 38% reported a medium level of stress.
Table 7
Caregiver Baseline Characteristics.
Caregiver Outcomes
Table 8 shows differences in caregiver outcomes between the study arms. There was no statistically significant difference between the 2 study arms in caregiver stress level or seeking social support.
Table 8
Caregiver Measures by Study Arm at 6 Months Compared With Baseline.
There was a statistically significant difference in reported emotional support at 6 months compared with baseline between the 2 study arms, with caregivers in the HCP Plus Peer Support arm reporting higher levels of emotional support (adjusted difference of 6.23 [95% CI, 1.52-10.94]; P = .01). No significant differences were found for informational support. There was a small difference in the PCS, with caregivers in the HCP Support arm reporting more preparedness at 6 months. Of note, the caregivers in this arm also had higher preparedness levels at baseline compared with those in the HCP Plus Peer Support arm.
Intermediate Outcomes
Impact on Self-efficacy and Self-care Behaviors
Table 9 shows changes in adapted UCOPD total and domain scores from baseline by study arm for each of the 3 study time points. After adjustment for baseline score, hospital site, and enrollment setting, there was a significant interaction between time and study arm for total UCOPD score (overall P < .001) and for the About COPD and Managing Symptoms domain scores (overall P < .001 for each). Looking individually at the 3 study time points for these outcomes using a Bonferroni-adjusted significance level of .05/3 = .0167, we found significant improvements from baseline to 6 months for total UCOPD score (difference of 3.91; 95% CI, 1.63-6.19; P = .001) and the About COPD (difference of 3.01; 95% CI, 1.55-4.74; P < .001) and Managing Symptoms domain scores (difference of 3.22; 95% CI, 1.18-5.27; P = .002). After adjustment for additional baseline patient characteristics, the improvement in the About COPD domain score remained statistically significant at this Bonferroni-adjusted significance level (Table 9).
Table 9
Change in UCOPD, Self-efficacy, and Self-care Behaviors.
There was no statistically significant difference in patient activation, as measured by the PAM score between the 2 study arms at 6 months (see Table 9A in Appendix A). There was a slightly higher number of participants in the HCP Plus Peer Support arm who had quit smoking or were attending a pulmonary rehabilitation program at 6 months compared with the HCP Support arm (6 vs 3 participants who had stopped smoking; 5 vs 3 participants attending a rehabilitation program), but those differences were not statistically significant.
Impact on Patient Perception of Support
Table 10 shows changes in PROMIS patient perception of support measures from baseline by study arm for each of the 3 study time points. After adjustment for baseline score, hospital site, and enrollment setting, there was a significant interaction between time and study arm for the Informational Support domain (overall P = .026). Using a Bonferroni-adjusted significance level of .05/3 = .0167, we saw no significant differences in change from baseline between the study arms for any individual time point. There was also a significant interaction between time and study arm for the Instrumental Support domain after adjustment for baseline score, hospital site, enrollment setting, and baseline patient characteristics (P = .023). We found a significantly larger improvement in instrumental support from baseline to 9 months in the HCP Plus Peer Support arm compared with the HCP Support arm (difference, 1.32; 95% CI, 0.30-2.34; P = .011).
Table 10
Change in PROMIS Support Measures.
Impact on Hope
Table 11 shows changes in hope, as measured by the HHI, by study arm at the 3 study time points (3 months, 6 months, 9 months). The HHI total scores range from 12 to 48, with higher scores indicating higher levels of hope. At 6 months, patients in the HCP Plus Peer Support arm had a mean improvement of 0.87 points in their HHI score compared with a mean decline of 1.18 points in the HCP Support arm (Table 11). There was a significant interaction between time point and study arm after adjustment for baseline score, hospital site, and enrollment setting (P = .001) and after additional adjustment for baseline patient characteristics (P < .001). Looking at the individual time points (with a Bonferroni-adjusted significance level of .0167), patients in the HCP Plus Peer Support arm had a significantly better change in HHI score from baseline to 6 months compared with those in the HCP Support arm (difference, 1.58; 95% CI, 0.42-2.74; P = .007) after adjustment for baseline score, hospital site, and enrollment setting. This difference was similar after additional adjustment for patient baseline characteristics (difference, 1.91; 95% CI, 0.87-2.96; P < .001). With the full set of adjusters, we also observed a significantly better but smaller change in HHI score from baseline to 3 months for the HCP Plus Peer Support arm than for the HCP Support arm (difference, 0.78; 95% CI, 0.41-1.15; P < .001).
Table 11
Changes in Hope Measured by the HHI.
Participant Experiences With the HCP Plus Peer Support Strategy
Survey responses were collected via phone from 49 participants out of 130 active HCP Plus Peer Support arm participants at the end of the study. On a scale of 1 to 10, where 1 was not useful and 10 was extremely useful, the participants rated the HCP session, BREATHE Pals calls, and get-together events. The mean ratings were 9.1 (SD, 1.6), 8.9 (SD, 1.4), and 9.3 (SD, 1.2) for the HCP session, BREATHE Pals calls, and get-together events, respectively. Table 12 shows themes and select quotes from participants' feedback on the BREATHE2 Peer Support program.
Table 12
Themes and Select Quotes From Participants' Feedback on the BREATHE2 Peer Support Program.
Intervention Implementation in the HCP Support and HCP Plus Peer Support arms
Table 13 shows the implementation of intervention services in each study arm by hospital site and enrollment setting. More than 99% of all participants in each study arm had an initial RCP session; 27.1% and 19.2% of participants in the HCP Support and HCP Plus Peer Support arms, respectively, contacted the RCP for a follow-up call during the 6-month follow-up period. Table 10A in Appendix A summarizes the themes from the RCP follow-up calls with participants.
Table 13
Intervention Implementation by Hospital Site and Enrollment Setting.
For participants randomly assigned to the HCP Plus Peer Support arm, the average number of peer support encounters (attending a BREATHE get-together or having a phone interaction with a BREATHE Pal) was 4.4 total encounters per participant. Based on our prespecified definition of adherence (having had at least 4 interactions with the peer program by either attending a BREATHE get-together or having a phone conversation with a BREATHE Pal), 67 (48.9%) participants in the HCP Plus Peer Support arm adhered to treatment, with the lowest adherence among JHBMC inpatients, particularly for attending group events. Reasons why participants reported not attending get-together events included being too sick to attend (marked as reason by 26% of respondents), having transportation issues (24%), and having other medical problems that were more important at the time. The odds of adherence to the intervention in the HCP Plus Peer Support arm was significantly lower for those enrolled from JHBMC inpatient (odds ratio [OR], 0.08; P < .001) and JHBMC outpatient (OR, 0.57; P = .031) settings than for HCGH inpatients.
The peer support program coordinator had a median of 3 calls per participant (ie, contacted 135 participants at least once during the study), with three-fourths of these contacts being of an administrative nature (mostly contacting patients about upcoming get-togethers) and 13% involving problem solving for transportation challenges (eg, connecting patients with Mobility Paratransit services, providing taxi coupons to participants for get-togethers). Table 11A in Appendix A summarizes the themes from the peer support program coordinator's calls with participants.
Using a prespecified adherence definition of having at least 4 interactions with peers via attending a get-together or having a phone conversation with a BREATHE Pal, we compared the baseline characteristics among participants who had adhered to the intervention and those who had low adherence. There were more participants with low income and education levels and higher comorbidities among the low-adherence group compared with the adherent group. Table 12A in Appendix A shows the baseline characteristics by adherence group.
Table 14 shows key patient outcomes by adherence group. For SGRQ total score, participants who adhered to the intervention had, on average, an improvement in HRQOL, while participants with low adherence had, on average, a reduction in HRQOL (mean differences at 6 months of −3.33 and 2.80, respectively). Participants who adhered to the intervention also had better HRQOL than those in in the HCP Support arm. This pattern holds for both SGRQ Activity and Impact domain scores. Additionally, acute-care use was lower among participants who adhered to the intervention compared with those who had low adherence and those in the HCP Support arm. This pattern holds for both all-cause and COPD-related acute-care events.
Table 14
Patient Outcomes by Intervention Reception.
Table 13A in Appendix A shows the intermediate patient outcomes by adherence group. There was an increase in emotional and informational support, patient activation, and UCOPD scores and a decrease in social isolation at 3 months and 6 months among participants who adhered to the intervention compared with those who had low adherence and those in the HCP Support arm.
HTE Analyses
Table 15 shows the results of HTE analyses for the primary outcome of HRQOL. We found a significant interaction between study arm and site (P < .001) in the total score for the SGRQ, with an adjusted difference in the primary outcome of −1.94 (95% CI, −2.18 to −1.70) at HCGH and 4.61 (95% CI, 2.82-6.41) at JHBMC (lower scores on the SGRQ indicate better HRQOL). No other significant interactions were found between study arm and other subgroup variables.
Table 15
Subgroup Analysis for the Mean Difference in HRQOL, as Measured by the SGRQ Total Score at 6 Months After Enrollment.
Table 14A and Table 15A in Appendix A show the patient baseline characteristics and the main study outcomes by hospital site and enrollment setting.
Discussion
In this trial, we compared the effectiveness of a dual strategy for provision of self-management support that incorporated HCP and peer support (HCP Plus Peer Support) with another that used only HCP support. We measured HRQOL over a 9-month period and found no significant differences between the study arms at 6 or 9 months. The HCP Plus Peer Support arm had less COPD-related acute-care events at 1, 3, and 6 months. The study findings are consistent with prior studies on self-management support interventions showing reductions in COPD-related acute-care use and marginal effects on HRQOL.86-89 Participants in the HCP Plus Peer Support arm had significant improvements in self-efficacy and hope levels compared with those in the HCP Support arm at the 6 -month time point. The effects on acute-care events, hope, and self-efficacy were observed only during the intervention period and were not seen at 9 months, suggesting that a longer intervention duration may be needed. Earlier studies on self-management and behavioral interventions for COPD, CHF, and other conditions have reported that longer intervention durations result in more lasting behavior changes and improvements in outcomes.88,90,91
In this trial, there was a higher percentage of participants who smoked or had CHF in the HCP Plus Peer Support arm than in the HCP Support arm (27.9% who smoked in the HCP Plus Peer Support arm compared with 21.4% in the HCP Support arm; 42.9% who had CHF in the HCP Plus Peer Support arm compared with 26.9% in the HCP Support arm). Although we have adjusted for these variables in our models, they indicate that at baseline, HCP Plus Peer Support arm participants were less healthy than HCP Support arm participants. Furthermore, in examining missingness of the QOL measure at 6 months between the 2 study arms, 18% of those observed vs 45% missing in the HCP Plus Peer Support arm were using continuous oxygen at baseline compared with 26% observed vs 34% missing in the HCP Support arm. This finding shows that the impact of missingness for this marker of severe COPD was greater in the HCP Plus Peer Support arm. Alternatively, it shows that the observed group assigned to the HCP Plus Peer Support arm was healthier than the missing group and perhaps had less to gain from the intervention. This hypothesis might have biased the comparison of the HCP Plus Peer and HCP Support arms toward the null.
The trial was conducted in real-life settings; although participants were encouraged to participate in all study interventions, they were not required to do so. To facilitate tailoring the intervention to patient preferences, the peer support program incorporated different modalities for provision of peer support, including group events and phone interactions. Counting both modalities of peer support provision, about half of the study participants had <4 interactions with peers. The number of interactions was particularly low among participants enrolled from the JHBMC inpatient population, from which approximately 40% of study participants were recruited. Notably, this population also had sicker patients with more comorbidities and lower socioeconomic and education levels. Though the number of peer interactions among participants from the JHBMC site by phone was similar between those participants recruited from the inpatient and outpatient settings, few participants recruited from the inpatient setting attended group events, despite intensive outreach efforts. Being too sick, having transportation challenges, and having other medical problems were reported as reasons for not attending group events, and these issues were more common among participants at the JHBMC site.
In this study, “expert patients” served as peer supporters, providing peer-to-peer support to help study participants self-manage COPD and minimize its impact on their QOL. The peer supporters had successfully stopped smoking and completed an acute pulmonary rehabilitation program; thus, they served as realistic role models who could provide a success story, hope, and evidence of a person's capacity to cope with COPD. Peer support has been shown to improve outcomes for several diseases and may reduce hospitalizations among patients with diabetes, mental health, and addiction problems.92-95 Peer support services are now commonly provided in mental health and addiction treatment programs, and payment mechanisms exist to pay for peer support services.96,97
To our knowledge, this is the first RCT to have studied the effect of peer support among patients with COPD. Generic (not condition-specific) programs to advance chronic disease self-management via structured education programs offered by peers have shown modest benefits on psychosocial outcomes, health behaviors, and utilization.42,98 These programs have mostly used passive recruitment approaches—mainly flyers and advertisements—and thus the reported outcomes likely represent treatment effects on that subset of patients who are interested, ready, and seeking such programs. In this study, however, we used systematic, proactive approaches for recruitment and enrolled participants during their inpatient stays once they expressed interest in joining the study. The tested population likely represents a broader population of patients with COPD at various stages of disease and variable readiness for receiving peer support and engaging in COPD self-management. The study's recruitment approach likely affected adherence rates to planned study interventions and limited our ability to show intervention effects based on ITT analysis. In an exploratory analysis comparing patient outcomes based on adherence group, participants who adhered to the HCP Plus Peer Support interventions had better QOL than did those who did not adhere at 6 months compared with baseline. They also had better QOL than the participants who were randomly assigned to the HCP Support arm. Similarly, we found better outcomes among participants who adhered to the HCP Plus Peer Support intervention (compared with those who did not adhere and those who were randomly assigned to the HCP Support arm), with less COPD-related and all-cause acute-care use, improved patient-reported emotional and informational support and UCOPD scores, higher patient activation, and decreased social isolation at 3 and 6 months compared with baseline. These findings are consistent with the anticipated changes according to the study's conceptual model (Figure 1).
Interestingly, caregivers in the HCP Plus Peer Support arm had significant increases in self-reported emotional support compared with those in the HCP Support arm, without significant changes in informational support or preparedness for caregiving. The study findings on the caregiver outcomes are limited by the low sample size, which reduces the study's power to detect statistically significant differences.
Generalizability
In this study, we enrolled a diverse group of study participants living in both urban and suburban locations. We had few exclusion criteria and recruited participants regardless of their comorbidities. Realizing that some patients who have COPD seek only emergency care services (because they are homebound, on continuous oxygen, or struggling with anxiety and depression) and are, therefore, unlikely to be recruited in clinic settings, we conducted recruitment activities in both inpatient and outpatient settings. We followed a systematic approach in informing all patients with COPD who were hospitalized, regardless of the reason for their hospitalization, about this study. Although many patients declined to participate because of other “pressing” comorbidities that interfered with their ability and interest in joining this study, those who joined had characteristics that were similar to people who were eligible and those who declined. Many joined, but then failed to participate in intervention activities.
Lessons Learned
We found no significant differences in HRQOL (study primary outcome) between the study arms. The participants randomly assigned to the HCP Plus Peer Support arm had fewer COPD-related acute-care events at 6 months after enrollment. Participation in peer support program activities was low, with an average number of peer encounters of 4.4 (SD, 4.2). The lower participation in the peer support program activities occurred despite having multiple options for peer conversations (group and 1:1, in person and by phone) and a systematic effort to address transportation barriers. Participation at the study site serving an urban population with low socioeconomic status and higher comorbidities (JHBMC) was lower than at the site serving a more affluent population (HCGH). Participation was also lower among participants who were recruited from inpatient settings. The recruitment strategies were proactive, and perhaps some inpatients were more hopeful about their ability to participate but were later overwhelmed by other health problems or barriers (as was reflected in responses on the Participant Experience Survey). Patients who enrolled in response to a letter from their provider sent to their home informing them about the program were more likely to engage in peer support activities.
Being too sick to attend, having transportation issues, or having medical problems that were more important at the time were the most reported reasons for not attending get-togethers. Though holding peer conversations by phone helped address some of these issues for some participants, it was not without its challenges. Both the BREATHE Pals and the peer support program coordinator reported that it was hard to reach many participants by phone. Also, some participants experience fatigue and feel short of breath when talking on the phone, and others have hearing difficulties. In addition, some have phone data plans with a limited number of minutes. For caregivers, some had difficulty participating because of conflicting job or family commitments, and it was not feasible to hold get-together events on multiple days and times to address time conflicts.
Subpopulation Considerations
We conducted an exploratory and hypothesis-generating HTE analysis on the study's primary outcome. The relative effectiveness of the 2 study interventions was the same in the subgroups for age, sex, oxygen use, and prior hospitalizations. The treatment effects were different, however, at the 2 study sites, with participants at the suburban, more affluent site having better outcomes. Of note, participation in program activities was also higher at this site (HCGH), with more participants having in-person interactions with peers.
Study Limitations
This study had several limitations:
- All study participants were recruited from within 1 health system; however, the study used a variety of sites and settings and recruited a diverse sample with few exclusion criteria.
- Participants were enrolled based on physician diagnosis of COPD without spirometry evidence.
- The measures of self-care behaviors were self-reported, and we did not have sufficient study power to detect differences in secondary or intermediate outcomes.
- Assessment of treatment effects was limited by low participation in peer support group activities, particularly among participants recruited from the JHBMC inpatient setting.
- We were able to enroll only a small number of family caregivers in the study.
- Per protocol, not all participants could have a 9-month outcome, because the study ended before they had reached 9 months.
- We could not reach a large number of participants at 6 months after discharge (31%); therefore, we could not measure their HRQOL. These participants were more likely to be on continuous oxygen therapy and may have had more socioeconomic challenges than those participants we were able to reach. We were, however, able to measure health care use outcomes for all participants in this study.
Recommendations for Future Research
Further research is needed to test peer support for patients at various stages of motivation and readiness and varying levels of disease severity. Studies are also needed to compare various modalities for peer support delivery, particularly those that exploit technology and reach patients who have more severe disease and other challenges. More research is needed on multimorbidity among patients with COPD and on how peer support can be provided for a variety of comorbidities.
Conclusions
In this RCT, we compared the effectiveness of HCP support alone vs HCP paired with peer support (HCP Plus Peer Support) for patients with COPD. We found no significant differences in QOL between the study arms; however, participants randomly assigned to the HCP Plus Peer Support arm had fewer COPD-related acute-care events and higher self-efficacy and hope scores than those in the HCP Support arm. Given the low participation in peer support activities and the substantial missing HRQOL outcome data, the study conclusions should be interpreted with caution. More research is needed to examine how peer support interventions can best be delivered and their treatment effects assessed among various patient subgroups.
References
- 1.
- Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. doi:10.7189/jogh.05-020415 [PMC free article: PMC4693508] [PubMed: 26755942] [CrossRef]
- 2.
- Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(46):938-943. [PubMed: 23169314]
- 3.
- Pfuntner A, Wier LM, Stocks C. Most Frequent Conditions in U.S. Hospitals, 2011. Statistical Brief No. 162. Agency for Healthcare Research and Quality; 2013. Accessed June 4, 2021. https://www
.ncbi.nlm .nih.gov/books/NBK169248/ [PubMed: 24228292] - 4.
- Jones R. Too little, too late—the patients' perspective on education for COPD. Chron Respir Dis. 2007;4(4):189-190. [PubMed: 18029430]
- 5.
- Rodgers S, Dyas J, Molyneux AW, Ward MJ, Revill SM. Evaluation of the information needs of patients with chronic obstructive pulmonary disease following pulmonary rehabilitation: a focus group study. Chron Respir Dis. 2007;4(4):195-203. [PubMed: 18029432]
- 6.
- Stellefson M, Tennant B, Chaney JD. A critical review of effects of COPD self-management education on self-efficacy. ISRN Public Health. 2012:152047. doi:10.5402/2012/152047 [CrossRef]
- 7.
- Press VG, Arora VM, Shah LM, et al. Teaching the use of respiratory inhalers to hospitalized patients with asthma or COPD: a randomized trial. J Gen Intern Med. 2012;27(10):1317-1325. doi:10.1007/s11606-012-2090-9 [PMC free article: PMC3445679] [PubMed: 22592354] [CrossRef]
- 8.
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930-938. doi:10.1016/j.rmed.2011.01.005 [PubMed: 21367593] [CrossRef]
- 9.
- Kessler R, Ståhl E, Vogelmeier C, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview-based study. Chest. 2006;130(1):133-142. doi:10.1378/chest.130.1.133 [PubMed: 16840393] [CrossRef]
- 10.
- Hernandez P, Balter M, Bourbeau J, Hodder R. Living with chronic obstructive pulmonary disease: a survey of patients' knowledge and attitudes. Respir Med. 2009;103(7):1004-1012. doi:10.1016/j.rmed.2009.01.018 [PubMed: 19269150] [CrossRef]
- 11.
- COPD Foundation releases groundbreaking COPE survey results: low patient awareness about COPD exacerbations poses barrier to effective management. Business Wire. June 17, 2014. Accessed July 30, 2020. https://www
.businesswire .com/news/home/20140617005140 /en/COPD-Foundation-Releases-Groundbreaking-COPE-Survey-Results - 12.
- Menezes AM, Landis SH, Han MK, et al. Continuing to Confront COPD International Surveys: comparison of patient and physician perceptions about COPD risk and management. Int J Chron Obstruct Pulmon Dis. 2015;10:159-172. doi:10.2147/COPD.S74315 [PMC free article: PMC4310342] [PubMed: 25653515] [CrossRef]
- 13.
- Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118(12):1415. doi:10.1016/j.amjmed.2005.07.059 [PubMed: 16378794] [CrossRef]
- 14.
- De Tratto K, Gomez C, Ryan CJ, Bracken N, Steffen A, Corbridge SJ. Nurses' knowledge of inhaler technique in the inpatient hospital setting. Clin Nurse Spec. 2014;28(3):156-160. [PubMed: 24714433]
- 15.
- De Godoy I, Nogueira DL, Godoy I. Nurses' knowledge and abilities gaps concerning health care of COPD patients: window for improvement. Eur Respir J. 2016;48(Suppl 60):PA1613. doi:10.1183/13993003.congress-2016.PA1613 [CrossRef]
- 16.
- Krishnan JA, Gussin HA, Prieto-Centurion V, Sullivan JL, Zaidi F, Thomashow BM. Integrating COPD into patient-centered hospital readmissions reduction programs. Chronic Obstr Pulm Dis. 2015;2(1):70-80. doi:10.15326/jcopdf.2.1.2014.0148 [PMC free article: PMC4410712] [PubMed: 25927076] [CrossRef]
- 17.
- Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526. doi:10.1016/S2213-2600(16)00094-1 [PubMed: 27185520] [CrossRef]
- 18.
- Brien SB, Lewith GT, Thomas M. Patient coping strategies in COPD across disease severity and quality of life: a qualitative study. NPJ Prim Care Respir Med. 2016;26:16051. doi:10.1038/npjpcrm.2016.51 [PMC free article: PMC5024412] [PubMed: 27629237] [CrossRef]
- 19.
- Miravitlles M, Pena-Longobardo LM, Oliva-Moreno J, Hidalgo-Vega A. Caregivers' burden in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:347-356. doi:10.2147/COPD.S76091 [PMC free article: PMC4334315] [PubMed: 25709429] [CrossRef]
- 20.
- Nakken N, Janssen DJ, van den Bogaart EH, et al. Informal caregivers of patients with COPD: home sweet home? Eur Respir Rev. 2015;24(137):498-504. doi:10.1183/16000617.00010114 [PMC free article: PMC9487697] [PubMed: 26324811] [CrossRef]
- 21.
- Pinto RA, Holanda MA, Medeiros MM, Mota RM, Pereira ED. Assessment of the burden of caregiving for patients with chronic obstructive pulmonary disease. Respir Med. 2007;101(11):2402-2408. doi:10.1016/j.rmed.2007.06.001 [PubMed: 17624751] [CrossRef]
- 22.
- Garlo K, O'Leary JR, Van Ness PH, Fried TR. Burden in caregivers of older adults with advanced illness. J Am Geriatr Soc. 2010;58(12):2315-2322. doi:10.1111/j.1532-5415.2010.03177.x [PMC free article: PMC3058825] [PubMed: 21087225] [CrossRef]
- 23.
- Simpson AC, Young J, Donahue M, Rocker G. A day at a time: caregiving on the edge in advanced COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:141-151. doi:10.2147/copd.s9881 [PMC free article: PMC2898087] [PubMed: 20631814] [CrossRef]
- 24.
- Trivedi RB, Bryson CL, Udris E, Au DH. The influence of informal caregivers on adherence in COPD patients. Ann Behav Med. 2012;44(1):66-72. doi:10.1007/s12160-012-9355-8 [PubMed: 22422104] [CrossRef]
- 25.
- Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(3):CD002990. doi:10.1002/14651858.CD002990.pub3 [PMC free article: PMC7004246] [PubMed: 24665053] [CrossRef]
- 26.
- Bourbeau J, van der Palen J. Promoting effective self-management programmes to improve COPD. Eur Respir J. 2009;33(3):461-463. doi:10.1183/09031936.00001309 [PubMed: 19251792] [CrossRef]
- 27.
- Pearson ML, Mattke S, Shaw R, Ridgely MS, Wiseman SH. Patient Self-management Support Programs: An Evaluation. Agency for Healthcare Research and Quality; November 2007. AHRQ Publication No. 08-0011. Accessed January 31, 2020. https://www
.ahrq.gov /sites/default/files /publications/files/ptmgmt.pdf - 28.
- Rennard S, Thomashow B, Crapo J, et al. Introducing the COPD Foundation Guide for Diagnosis and Management of COPD, recommendations of the COPD Foundation. COPD. 2013;10(3):378-389. [PubMed: 23713598]
- 29.
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365. doi:10.1164/rccm.201204-0596PP [PubMed: 22878278] [CrossRef]
- 30.
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191. doi:10.7326/0003-4819-155-3-201108020-00008 [PubMed: 21810710] [CrossRef]
- 31.
- Bourbeau J, van der Palen J. Promoting effective self-management programmes to improve COPD. Eur Respir J. 2009;33(3):461-463. doi:10.1183/09031936.00001309 [PubMed: 19251792] [CrossRef]
- 32.
- Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):549-555. doi:10.1513/pats.200709-148ET [PMC free article: PMC2645334] [PubMed: 18453370] [CrossRef]
- 33.
- Nici L, ZuWallack R; American Thoracic Society Subcommittee on Integrated Care of the COPD Patient. An official American Thoracic Society workshop report: the integrated care of the COPD patient. Proc Am Thorac Soc. 2012;9(1):9-18. doi:10.1513/pats.201201-014ST [PubMed: 22421582] [CrossRef]
- 34.
- Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J Jr; Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284(21):2762-2770. doi:10.1001/jama.284.21.2762 [PubMed: 11105183] [CrossRef]
- 35.
- Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894-903. doi:10.7326/0003-4819-144-12-200606200-00006 [PubMed: 16785478] [CrossRef]
- 36.
- Foster JA, Yawn BP, Maziar A, Jenkins T, Rennard SI, Casebeer L. Enhancing COPD management in primary care settings. MedGenMed. 2007;9(3):24. Accessed June 4, 2021. https://www
.ncbi.nlm .nih.gov/pmc/articles/PMC2100091/ [PMC free article: PMC2100091] [PubMed: 18092030] - 37.
- Salinas GD, Williamson JC, Kalhan R, et al. Barriers to adherence to chronic obstructive pulmonary disease guidelines by primary care physicians. Int J Chron Obstruct Pulmon Dis. 2011;6:171-179. doi:10.2147/COPD.S16396 [PMC free article: PMC3064423] [PubMed: 21468169] [CrossRef]
- 38.
- Taylor SJ, Sohanpal R, Bremner SA, et al. Self-management support for moderate-to-severe chronic obstructive pulmonary disease: a pilot randomised controlled trial. Br J Gen Pract. 2012;62(603):e687-e695. doi:10.3399/bjgp12X656829 [PMC free article: PMC3459776] [PubMed: 23265228] [CrossRef]
- 39.
- Campos MA, Alazemi S, Zhang G, Wanner A, Sandhaus RA. Effects of a disease management program in individuals with alpha-1 antitrypsin deficiency. COPD. 2009;6(1):31-40. [PubMed: 19229706]
- 40.
- Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39(11):1217-1223. [PubMed: 11606875]
- 41.
- Clark NM, Becker MH, Janz NK, Lorig K, Rakowski W, Anderson L. Self-management of chronic disease by older adults: a review and questions for research. J Aging Health. 1991;3(1):3-27.
- 42.
- Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37(1):5-14. [PubMed: 10413387]
- 43.
- Tang TS, Ayala GX, Cherrington A, Rana G. A review of volunteer-based peer support interventions in diabetes. Diabetes Spectrum. 2011;24(2):85-98.
- 44.
- Fisher EB, Boothroyd RI, Coufal MM, et al. Peer support for self-management of diabetes improved outcomes in international settings. Health Aff (Millwood). 2012;31(1):130-139. doi:10.1377/hlthaff.2011.0914 [PMC free article: PMC4128379] [PubMed: 22232103] [CrossRef]
- 45.
- Long JA, Jahnle EC, Richardson DM, Loewenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med. 2012;156(6):416-424. doi:10.7326/0003-4819-156-6-201203200-00004 [PMC free article: PMC3475415] [PubMed: 22431674] [CrossRef]
- 46.
- Parent N, Fortin F. A randomized, controlled trial of vicarious experience through peer support for male first-time cardiac surgery patients: impact on anxiety, self-efficacy expectation, and self-reported activity. Heart Lung. 2000;29(6):389-400. [PubMed: 11080319]
- 47.
- Fisher EB, Coufal MM, Parada H, et al. Peer support in health care and prevention: cultural, organizational, and dissemination issues. Annu Rev Public Health. 2014;35:363-383. doi:10.1146/annurev-publhealth-032013-182450 [PubMed: 24387085] [CrossRef]
- 48.
- Crawford S, Bath N. Peer support models for people with a history of injecting drug use undertaking assessment and treatment for hepatitis C virus infection. Clin Infect Dis. 2013;57(Suppl 2):S75-S79. doi:10.1093/cid/cit297 [PubMed: 23884070] [CrossRef]
- 49.
- Halding AG, Wahl A, Heggdal K. ‘Belonging’. Patients' experiences of social relationships during pulmonary rehabilitation. Disabil Rehabil. 2010;32(15):1272-1280. doi:10.3109/09638280903464471 [PMC free article: PMC2942866] [PubMed: 20156041] [CrossRef]
- 50.
- Philis-Tsimikas A, Fortmann A, Lleva-Ocana L, Walker C, Gallo LC. Peer-led diabetes education programs in high-risk Mexican Americans improve glycemic control compared with standard approaches: a Project Dulce promotora randomized trial. Diabetes Care. 2011;34(9):1926-1931. doi:10.2337/dc10-2081 [PMC free article: PMC3161298] [PubMed: 21775748] [CrossRef]
- 51.
- Dennis CL. Peer support within a health care context: a concept analysis. Int J Nurs Stud. 2003;40(3):321-332. [PubMed: 12605954]
- 52.
- Bandura A, Adams NE, Beyer J. Cognitive processes mediating behavioral change. J Pers Soc Psychol. 1977;35(3):125-139. [PubMed: 15093]
- 53.
- Sokol R, Fisher E. Peer support for the hardly reached: a systematic review. Am J Public Health. 2016;106(7):e1-e8. doi:10.2105/AJPH.2016.303180 [PMC free article: PMC4984766] [PubMed: 27196645] [CrossRef]
- 54.
- Harris S. COPD and coping with breathlessness at home: a review of the literature. Br J Community Nurs. 2007;12(9):411-415. [PubMed: 18026004]
- 55.
- Lee RN, Graydon JE, Ross E. Effects of psychological well-being, physical status, and social support on oxygen-dependent COPD patients' level of functioning. Res Nurs Health. 1991;14(5):323-328. [PubMed: 1891618]
- 56.
- Grodner S, Prewitt LM, Jaworsk BA, Myers R, Kaplan RM, Ries AL. The impact of social support in pulmonary rehabilitation of patients with chronic obstructive pulmonary disease. Ann Behav Med. 1996;18(3):139-145. [PubMed: 24203764]
- 57.
- Wakabayashi R, Motegi T, Yamada K, Ishii T, Gemma A, Kida K. Presence of in-home caregiver and health outcomes of older adults with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2011;59(1):44-49. [PubMed: 21198462]
- 58.
- Deek H, Hamilton S, Brown N, et al. Family-centred approaches to healthcare interventions in chronic diseases in adults: a quantitative systematic review. J Adv Nurs. 2016;72(5):968-979. [PubMed: 26751971]
- 59.
- Pamungkas RA, Chamroonsawasdi K, Vatanasomboon P. A systematic review: family support integrated with diabetes self-management among uncontrolled type II diabetes mellitus patients. Behav Sci (Basel). 2017;7(3):62. doi:10.3390/bs7030062 [PMC free article: PMC5618070] [PubMed: 28914815] [CrossRef]
- 60.
- Aboumatar H, Naqibuddin M, Neiman J, et al. Methodology and baseline characteristics of a randomized controlled trial testing a health care professional and peer-support program for patients with chronic obstructive pulmonary disease: the BREATHE2 study. Contemp Clin Trials. 2020;94:106023. doi:10.1016/j.cct.2020.106023 [PubMed: 32360887] [CrossRef]
- 61.
- Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946. doi:10.1183/09031936.04.00014304 [PubMed: 15219010] [CrossRef]
- 62.
- Riegel B, Carlson B. Is individual peer support a promising intervention for persons with heart failure? J Cardiovasc Nurs. 2004;19(3):174-183. [PubMed: 15191260]
- 63.
- Perry E, Swartz J, Brown S, Smith D, Kelly G, Swartz R. Peer mentoring: a culturally sensitive approach to end-of-life planning for long-term dialysis patients. Am J Kidney Dis. 2005;46(1):111-119. [PubMed: 15983964]
- 64.
- Foster G, Taylor SJC, Eldridge SE, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev. 2007;(4):CD005108. doi:10.1002/14651858.CD005108.pub2 [PubMed: 17943839] [CrossRef]
- 65.
- Funnell MM. Peer-based behavioural strategies to improve chronic disease self-management and clinical outcomes: evidence, logistics, evaluation considerations and needs for future research. Fam Pract. 2010;27(Suppl 1):i17-i22. [PMC free article: PMC2873176] [PubMed: 19509083]
- 66.
- Alberto J, Joyner B. Hope, optimism, and self-care among Better Breathers Support Group members with chronic obstructive pulmonary disease. Appl Nurs Res. 2008;21(4):212-217. [PubMed: 18995163]
- 67.
- Embuldeniya G, Veinot P, Bell E, et al. The experience and impact of chronic disease peer support interventions: a qualitative synthesis. Patient Educ Couns. 2013;92(1):3-12. doi:10.1016/j.pec.2013.02.002 [PubMed: 23453850] [CrossRef]
- 68.
- Kohler CL, Fish L, Greene PG. The relationship of perceived self-efficacy to quality of life in chronic obstructive pulmonary disease. Health Psychol. 2002;21(6):610-614. [PubMed: 12433014]
- 69.
- Arnold R, Ranchor AV, DeJongste MJL et al. The relationship between self-efficacy and self-reported physical functioning in chronic obstructive pulmonary disease and chronic heart failure. Behav Med. 2005;31(3):107-115. [PubMed: 16252622]
- 70.
- Davis AH, Carrieri-Kohlman V, Janson SL, et al. Effects of treatment on two types of self-efficacy in people with chronic obstructive pulmonary disease. J Pain Symptom Manage. 2006;32:60-70. [PubMed: 16824986]
- 71.
- Jones PW, Quirk FH, Baveystock CM. The St. George's Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25-37. [PubMed: 1759018]
- 72.
- Ader DN. Developing the patient-reported outcomes measurement information system (PROMIS). Med Care. 2007;45(5):S1-S2. doi:10.1097/01.mlr.0000260537.45076.74 [PMC free article: PMC2829758] [PubMed: 17443116] [CrossRef]
- 73.
- Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179-1194. doi:10.1016/j.jclinepi.2010.04.011 [PMC free article: PMC2965562] [PubMed: 20685078] [CrossRef]
- 74.
- Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the Patient Activation Measure. Health Serv Res. 2005;40(6 Pt 1):1918-1930. [PMC free article: PMC1361231] [PubMed: 16336556]
- 75.
- Herth K. Abbreviated instrument to measure hope: development and psychometric evaluation. J Adv Nurs. 1992;17(10):1251-1259. [PubMed: 1430629]
- 76.
- O'Neill B, Cosgrove D, MacMahon J, McCrum-Gardner E, Bradley JM. Assessing education in pulmonary rehabilitation: the Understanding COPD (UCOPD) questionnaire. COPD. 2012;9(2):166-174. [PubMed: 22409266]
- 78.
- Zwicker D. Preparedness for Caregiving Scale. Try This: Best Practices in Nursing Care to Older Adults. 2018;(28). Accessed July 30, 2020. https://hign
.org/sites /default/files/2020-06 /Try_This_General_Assessment_28.pdf - 79.
- Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, O'Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41(5):652-657. doi:10.1093/geront/41.5.652 [PubMed: 11574710] [CrossRef]
- 80.
- Vitaliano PP, Russo J, Carr JE, Maiuro RD, Becker J. The Ways of Coping checklist: revision and psychometric properties. Multivariate Behav Res. 1985;20(1):3-26. [PubMed: 26776273]
- 81.
- Thornton M, Travis SS. Analysis of the reliability of the modified Caregiver Strain Index. J Gerontol B Psychol Sci Soc Sci. 2003;58(2):S127-S132. doi:10.1093/geronb/58.2.s127 [PubMed: 12646602] [CrossRef]
- 82.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8 [PubMed: 3558716] [CrossRef]
- 83.
- Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23(5):561-566. doi:10.1007/s11606-008-0520-5 [PMC free article: PMC2324160] [PubMed: 18335281] [CrossRef]
- 84.
- Clinical public use data requests. Maryland Health Services Cost Review Commission. Accessed January 31, 2020. https://hscrc
.state.md .us/Pages/hsp-data-request.aspx - 85.
- Research and quality improvement. Chesapeake Regional Information System for Our Patients (CRISP). August 24, 2021. https://www
.crisphealth .org/resources/research-and-quality-improvement/ - 86.
- Effing T, Monninkhof EM, van der Valk PD, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;(4):CD002990. doi:10.1002/14651858.CD002990.pub2 [PubMed: 17943778] [CrossRef]
- 87.
- Tan JY, Chen JX, Liu XL, et al. A meta-analysis on the impact of disease-specific education programs on health outcomes for patients with chronic obstructive pulmonary disease. Geriatr Nurs. 2012;33(4):280-296. [PubMed: 22595334]
- 88.
- Jonkman NH, Westland H, Trappenburg JCA, et al. Characteristics of effective self-management interventions in patients with COPD: individual patient data meta-analysis. Eur Respir J. 2016;48(1):55-68. doi:10.1183/13993003.01860-2015 [PubMed: 27126694] [CrossRef]
- 89.
- Lenferink A, Brusse-Keizer M, van der Valk PD, et al. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;8(8):CD011682. doi:10.1002/14651858.CD011682.pub2 [PMC free article: PMC6483374] [PubMed: 28777450] [CrossRef]
- 90.
- Fjeldsoe B, Neuhaus M, Winkler E, Eakin E. Systematic review of maintenance of behavior change following physical activity and dietary interventions. Health Psychol. 2011;30(1):99-109. [PubMed: 21299298]
- 91.
- Göhler A, Januzzi JL, Worrell SS, et al. A systematic meta-analysis of the efficacy and heterogeneity of disease management programs in congestive heart failure. J Card Fail. 2006;12(7):554-567. [PubMed: 16952790]
- 92.
- Davidson L, Chinman M, Sells D, Rowe M. Peer support among adults with serious mental illness: a report from the field. Schizophr Bull. 2006;32(3):443-450. [PMC free article: PMC2632237] [PubMed: 16461576]
- 93.
- Chinman M, George P, Dougherty RH, et al. Peer support services for individuals with serious mental illnesses: assessing the evidence. Psychiatr Serv. 2014;65(4):429-441. doi:10.1176/appi.ps.201300244 [PubMed: 24549400] [CrossRef]
- 94.
- Latkin CA, Sherman S, Knowlton A. HIV prevention among drug users: outcome of a network-oriented peer outreach intervention. Health Psychol. 2003;22(4):332-339. [PubMed: 12940388]
- 95.
- Dennis CL, Hodnett E, Kenton L, et al. Effect of peer support on prevention of postnatal depression among high risk women: multisite randomised controlled trial. BMJ. 2009;338:a3064. doi:10.1136/bmj.a3064 [PMC free article: PMC2628301] [PubMed: 19147637] [CrossRef]
- 96.
- Davidson L, Bellamy C, Guy K, Miller R. Peer support among persons with severe mental illnesses: a review of evidence and experience. World Psychiatry. 2012;11(2):123-128. doi:10.1016/j.wpsyc.2012.05.009 [PMC free article: PMC3363389] [PubMed: 22654945] [CrossRef]
- 97.
- Peers. Substance Abuse and Mental Health Services Administration (SAMHSA). Accessed August 24, 2021. https://www
.samhsa.gov /brss-tacs/recovery-support-tools /peers - 98.
- Brady TJ, Murphy L, O'Colmain BJ, et al. A meta-analysis of health status, health behaviors, and health care utilization outcomes of the chronic disease self-management program. Prev Chronic Dis. 2013;10:120112. doi:10.5888/pcd10.120112 [PMC free article: PMC3547675] [PubMed: 23327828] [CrossRef]
Acknowledgments
We are deeply grateful to all members of our patient and family partners group for their valuable contributions to the development of the BREATHE2 study.
We also thank all our study participants and study partners, including Johns Hopkins Bayview Medical Center (JHBMC), Howard County General Hospital (HCGH), and Johns Hopkins Community Physicians. We highly appreciate the contributions and support of the Respiratory Care group and the Pulmonary Rehabilitation programs at JHBMC and HCGH.
The BREATHE2 Study was possible through funding from PCORI (award no. CDR-1507-31247). The opinions in this publication are solely the responsibility of the authors and do not necessarily represent the views of PCORI, its Board of Governors, or the Methodology Committee.
Research reported in this report was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (CDR-1507-31247). Further information available at: https://www.pcori.org/research-results/2016/comparing-self-management-program-and-without-peer-support-improve-quality
Appendices
Appendix A.
Additional Tables (PDF, 606K)
Table 2A. Examples of Engagement Impact (PDF, 117K)
Table 5A. Demographic characteristics of eligible patients and those who enrolled or declined to participate (PDF, 202K)
Table 8A. Mean change in HRQoL as measured by SGRQ from baseline to 6 and 9 months post-enrollment (PDF, 146K)
Table 9A. Patient Activation Scores (PDF, 119K)
Appendix B.
Recommended Steps for Implementing the BREATHE2 Program (PDF, 324K)
Appendix C.
BREATHE2 Educational Materials (PDF, 1.4M)
Suggested citation:
Aboumatar H, Naqibuddin M, Saunders J, et al. (2021). Comparing a Self-Management Program with and without Peer Support to Improve Quality of Life for Patients with COPD. Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/06.2021.CDR.150731247
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- Suicidal Ideation.[StatPearls. 2024]Suicidal Ideation.Harmer B, Lee S, Rizvi A, Saadabadi A. StatPearls. 2024 Jan
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Comparing Self-Management Programs with and without Peer Support among Patients with Chronic Obstructive Pulmonary Disease: A Clinical Trial.[Ann Am Thorac Soc. 2022]Comparing Self-Management Programs with and without Peer Support among Patients with Chronic Obstructive Pulmonary Disease: A Clinical Trial.Aboumatar H, Garcia Morales EE, Jager LR, Naqibuddin M, Kim S, Saunders J, Bone L, Linnell J, McBurney M, Neiman J, et al. Ann Am Thorac Soc. 2022 Oct; 19(10):1687-1696.
- Review Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease.[Cochrane Database Syst Rev. 2017]Review Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease.Lenferink A, Brusse-Keizer M, van der Valk PD, Frith PA, Zwerink M, Monninkhof EM, van der Palen J, Effing TW. Cochrane Database Syst Rev. 2017 Aug 4; 8(8):CD011682. Epub 2017 Aug 4.
- Review Does Working with a Health Coach Help Patients with COPD Improve Their Quality of Life and Breathe Easier?[ 2019]Review Does Working with a Health Coach Help Patients with COPD Improve Their Quality of Life and Breathe Easier?Thom DH, Su G, Hessler D, Willard-Grace R. 2019 Sep
- Comparing a Self-Management Program with and without Peer Support to Improve Qua...Comparing a Self-Management Program with and without Peer Support to Improve Quality of Life for Patients with COPD
Your browsing activity is empty.
Activity recording is turned off.
See more...
