NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Valacyclovir is a nucleoside analogue antiviral agent and prodrug of acyclovir which is used in therapy of herpes simplex and varicella-zoster virus infections. Valacyclovir has been associated with rare instances mild, clinically apparent liver injury.
Background
Valacyclovir (val" ay sye' kloe vir), which is sometimes spelled valaciclovir, is an acyclic purine nucleoside analogue, the L-valyl ester prodrug of acyclovir that once absorbed, is rapidly converted to acyclovir, its active moiety. Valacyclovir has greater oral bioavailability than acyclovir and has similar activity against herpes viruses, including herpes simplex 1 and 2, cytomegalovirus, Epstein-Barr virus and varicella-zoster. Once converted to acyclovir, the drug is phosphorylated intracellularly by viral kinases. The resultant triphosphate competes with guanosine for incorporation into viral DNA, blocking viral DNA polymerase activity. Because its activation requires the presence of viral kinases, valacyclovir is only activated in virally infected cells. Valacyclovir is indicated for therapy of mucocutaneous and genital herpes simplex infections, both type 1 and 2 and for herpes zoster. Valacyclovir was approved for use in the United States in 1995 and is widely used in the treatment and prophylaxis of genital and mucocutaneous herpes simplex infection. Valacyclovir is available as capsules of 500 mg and 1000 mg generically and under the brand name of Valtrex. The usually recommended dose in adults is 500 to 1000 mg once or twice daily. Side effects are uncommon, but include headache, dizziness and gastrointestinal upset. Rare but potentially severe adverse events include renal dysfunction, CNS effects and severe thrombocytopenia.
Hepatotoxicity
Oral therapy with valacyclovir is associated with a low rate of mild-to-moderate serum aminotransferase elevations, but these abnormalities are usually asymptomatic and self-limited even with continuation of therapy. Complicating the attribution of liver test abnormalities to valacyclovir therapy is the fact that enzyme elevations are not uncommon during the course of varicella-zoster infection (both chickenpox and shingles) and can progress to clinically apparent hepatitis and even acute liver failure. Clinically apparent liver disease due to valacyclovir itself is rare, but isolated reports have been published. The time to onset was short (1 to 2 weeks) and the course mild, with few symptoms and rapid resolution (Case 1). The pattern of liver injury described was mixed hepatocellular-cholestatic. Immunoallergic features and autoantibodies were absent.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
After absorption, valacyclovir is converted to acyclovir by the liver, which is metabolized intracellularly in viral infected cells and is excreted largely unchanged by the kidneys. Valacyclovir is not activated in cells without viral kinases, perhaps accounting for the absence or rarity of hepatic injury.
Outcome and Management
No instances of acute liver failure or chronic liver injury have been linked to valacyclovir use. The liver injury associated with valacyclovir is usually mild and resolves rapidly. There is no information on possible cross sensitivity of hepatic injury among the various nucleoside analogues used to treat herpes virus infections, but valacyclovir is a prodrug of acyclovir which is its active moiety.
Drug Class: Antiviral Agents
Other Antiviral Agents for Herpes Virus Infections: Acyclovir, Cidofovir, Famciclovir, Foscarnet, Ganciclovir, Letermovir, Valganciclovir
CASE REPORT
Case 1. Mild acute hepatitis with jaundice after valacyclovir therapy.(1)
A 71 year old woman with shingles was treated with valacyclovir (3 g daily) for 7 days and developed abdominal and back pains by the end of therapy, leading to hospitalization 2 days later. She was also taking acetaminophen for pain in doses up to 3 g daily. She had no history of liver disease, risk factors for hepatitis or alcohol abuse. She was taking thyroid hormone for hypothyroidism, but no other medications. She had a history of cholecystectomy. On admission, she did not have fever or rash and the abdominal pain was attributed to back strain. Laboratory tests showed elevations in serum ALT and alkaline phosphatase with mild hyperbilirubinemia (Table), but no elevations in serum amylase or creatinine. Valacyclovir was stopped. Tests for hepatitis A, B and C were negative as were autoantibodies. An ultrasound showed a prominent common bile duct (9 mm), but subsequent ERCP did not show obstruction or biliary stones. After 5 days, acetaminophen was stopped and other analgesics were substituted. Two weeks after initial presentation, the liver test abnormalities had resolved.
Key Points
| Medication: | Valacyclovir (3 g daily) |
|---|---|
| Pattern: | Mixed (R=3.5) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 1 week |
| Recovery: | Complete in 2 weeks |
| Other medications: | Acetaminophen, thyroid hormone |
Laboratory Values
Comment
A mild case of liver injury with a mixed pattern of serum enzyme elevations arising by the end of a one-week course of valacyclovir. While she was also taking acetaminophen in somewhat high doses, the pattern of enzyme elevations and absence of renal dysfunction were atypical for acetaminophen toxicity. The possibility of varicella-zoster induced hepatitis should also be considered.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Valacyclovir – Generic, Valtrex®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
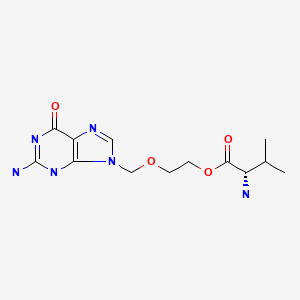
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Valacyclovir | 124832-26-4 | C13-H20-N6-O4 |
|
CITED REFERENCE
- 1.
- Renkes P, Trechot P, Blain H. Valaciclovir-induced hepatitis. Acta Clin Belg. 1999;54:17–8. [PubMed: 10192972]
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2020
- Zimmerman HJ. Antiviral agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 621-3.(Expert review of antiviral agents and liver injury published in 1999; valacyclovir is not discussed, but acyclovir is said to have not caused "overt hepatic injury").
- Núñez M. Herpesviridae treatment. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 512-3.(Review of hepatotoxicity of antiviral agents; mentions that valacyclovir has been associated with serum enzyme elevations during oral therapy).
- Acosta EP. Antiviral agents(nonretroviral). In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1105-18.(Textbook of pharmacology and therapeutics).
- Bodsworth NJ, Crooks RJ, Borelli S, Vejlsgaard G, Paavonen J, Worm A-M, Uexkull N, et al. International Valaciclovir HSV Study Group. Genitourin Med. 1997;73:110–6. [PMC free article: PMC1195783] [PubMed: 9215092](Among 999 patients randomized at 48 sites to treatment with acyclovir or valacyclovir for 5 days for recurrent HSV infection, there was equivalent efficacy and "no clinically important changes from screening in any clinical chemistry variable").
- Dits H, Frans E, Wilmer A, Van Ranst M, Fevery J, Bobbaers H. Varicella-zoster virus infection associated with acute liver failure. Clin Infect Dis. 1998;27:209–10. [PubMed: 9675478](30 year old previously healthy man exposed to a child with chicken pox developed fever, vesicular rash and progressive liver disease [bilirubin 0.82 mg/dL, ALT 27 rising to 1650 U/L, Alk P not given, INR 1.02 rising to 10.44], with multiorgan failure and death, culture of liver showing varicella zoster virus).
- Renkes P, Trechot P, Blain H. Valaciclovir-induced hepatitis. Acta Clin Belg. 1999;54:17–8. [PubMed: 10192972](71 year old woman with shingles developed abdominal pain 7 days after starting valacyclovir and acetaminophen [3 g/day] with ALT 376 U/L, Alk P 246 U/L and bilirubin 3.3 mg/dL, resolving within 2 weeks of stopping: Case 1).
- Ormrod D, Scott LJ, Perrry CM. Valaciclovir: a review of its long term utility in the management of genital herpes simplex virus and cytomegalovirus infections. Drugs. 2000;59:839–63. [PubMed: 10804039](Review of efficacy and safety of long term valacyclovir use; rates of side effects are similar in frequency to those in patients on acyclovir and on placebo; a single case of hepatitis due to valacyclovir has been reported in abstract form).
- Mantadakis E, Anagnostatou N, Danilatou V, Markaki EA, Spanaki AM, Briassoulis G, Kalmanti M. Fulminant hepatitis due to varicella zoster virus in a girl with acute lymphoblastic leukemia in remission: report of a case and review. J Pediatr Hematol Oncol. 2005;27:551–3. [PubMed: 16217259](4 year old girl on dexamethasone while in remission from acute leukemia developed abdominal pain followed by worsening liver tests and progressive liver failure [bilirubin 0.9 rising to 2.6 mg/dL, ALT 3790 U/L, INR 2.68 rising to 4.38], autopsy showing massive liver necrosis and varicella zoster virus).
- Brantley JS, Hicks L, Sra K, Tyring SK. Valacyclovir for the treatment of genital herpes. Expert Rev Anti Infect Ther. 2006;4:367–76. [PubMed: 16771614](Review of chemistry, pharmacology, efficacy and safety of valacyclovir in treating genital herpes; side effect profile is similar to acyclovir with no significant drug interactions; no mention of ALT elevations or hepatotoxicity).
- Drugs for non-HIV viral infections. Treat Guidel Med Lett. 2007;5:59–70. [PubMed: 17565338](Review of status of non-antiretroviral antiviral agents for prevention and treatment of herpes, varicella-zoster, cytomegalovirus, influenza A and B, and hepatitis B and C; no mention of liver related side effects for valacyclovir).
- Breuer J, Whitley R. Varicella zoster virus: natural history and current therapies of varicella and herpes zoster. Herpes. 2007;14 Suppl 2:25–9. [PubMed: 17939892](Review of use of antiviral agents in herpes virus infections).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 8 were attributed to antiviral agents including one due to valacyclovir; 50 year old woman with shingles developed jaundice 10 days after stopping a 2 week course of valacyclovir [peak bilirubin 6.4 mg/dL, ALT 785 U/L, Alk P 666 U/L]).
- Mizoguchi F, Nakamura S, Iwai H, Kubota T, Miyasaka N. Varicella-zoster virus hepatitis in polymyositis. Mod Rheumatol. 2008;18:301–5. [PubMed: 18360803](31 year old woman with polymyositis on prednisolone and methotrexate developed fever, pustular rash and hepatitis [bilirubin not given, ALT 230 rising to 4795 U/L], resolving with acyclovir, prednisolone and plasmapheresis therapy).
- Drebber U, Preuss SF, Kasper HU, Wieland U, Dienes HP. Postoperative fulminant varicella zoster virus hepatitis with fatal outcome: a case report. Z Gastroenterol. 2008;46:45–7. [PubMed: 18188815](49 year old man with laryngeal cancer developed liver injury 15 days after laryngectomy [bilirubin and Alk P not given, ALT 1332 U/L], with progressive hepatic failure and death; autopsy showing coagulative necrosis and viral inclusions in adjacent hepatocytes, typical of varicella zoster hepatitis).
- Al-Hamoudi WK. Severe autoimmune hepatitis triggered by varicella zoster infection. World J Gastroenterol. 2009;15:1004–6. [PMC free article: PMC2653401] [PubMed: 19248202](23 year old man developed jaundice within a month and while recovering from chicken pox [bilirubin 24.8 mg/dL, ALT 1066 U/L, Alk P 185 U/L, IgG 2050 mg/dL, ANA negative], responding to prednisone, but with relapses when drug was stopped or dose reduced).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, 4 of which were due to antiretroviral agents, but none were attributed to an antiherpes virus agent).
- Antiviral drugs. Treat Guidel Med Lett. 2013;11(127):19–30. [PubMed: 23459414](Review of safety and efficacy of valacyclovir treatment and prophylaxis against varicella and herpes zoster infections; mentions that it is converted to acyclovir and its side effects are likely to be similar; does not specifically mention liver injury).
- Prado NMBL, Messias GC, Santos GO Junior, Nunes VS, Schinonni MI, Paraná R. Prospective monitoring of drug use: drug-induced liver injury in a primary healthcare center. Arq Gastroenterol. 2019;56:390–3. [PubMed: 31721973](Among 149 patients followed in a primary care center in Brazil, three were suspected to have developed liver injury due to a medication including nimesulide, budesonide and valacyclovir marked by moderate elevations in ALT levels [176-182 U/L], normal Alk P [46-52 U/L], normal bilirubin [0.3-0.4 mg/dL] and no symptoms, and resolving rapidly on drug discontinuation).
- Burton MJ, Penman A, Sunesara I, McGuire BM, Hook EW 3rd. A pilot study examining the safety and tolerability of valacyclovir in veterans with hepatitis C virus/herpes simplex virus type 2 coinfection. Am J Med Sci. 2014;348(6):455–9. [PubMed: 25163019](Among 30 patients with chronic hepatitis C and herpes simplex 2 infection treated with valacyclovir [1 g] or placebo twice times daily for 8 weeks followed by a washout period and then cross over to the alternative therapy, there was no toxicity and ALT levels declined slightly [-8% to -10%] as did HCV RNA levels [- 24%] during valacyclovir therapy).
- Antiviral drugs for varicella-zoster virus and herpes simplex virus infections. Med Lett Drugs Ther. 2018;60(1556):153–7. [PubMed: 30383727](Concise review of the therapy of varicella-zoster and herpes simplex infections, mentions that valacyclovir is a pro-drug of acyclovir and has similar efficacy and side effects but better absorption and pharmacokinetics; common adverse event include gastrointestinal disturbances, headache, malaise; no mention of ALT elevations or hepatotoxicity).
- Kennedy PGE, Gershon AA. Clinical features of varicella-zoster virus infection. Viruses. 2018;10:609. [PMC free article: PMC6266119] [PubMed: 30400213](Review of the clinical features of varicella-zoster infections, including chicken pox and herpes zoster [reactivation] mentions that hepatitis can accompany varicella-zoster infections).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Valacyclovir in the treatment of herpes simplex, herpes zoster, and other viral infections.[J Cutan Med Surg. 2003]Review Valacyclovir in the treatment of herpes simplex, herpes zoster, and other viral infections.Wu JJ, Brentjens MH, Torres G, Yeung-Yue K, Lee P, Tyring SK. J Cutan Med Surg. 2003 Sep-Oct; 7(5):372-81. Epub 2003 Sep 24.
- Review Valacyclovir for the treatment of genital herpes.[Expert Rev Anti Infect Ther. 2...]Review Valacyclovir for the treatment of genital herpes.Brantley JS, Hicks L, Sra K, Tyring SK. Expert Rev Anti Infect Ther. 2006 Jun; 4(3):367-76.
- Review Valacyclovir versus acyclovir for the treatment of herpes zoster ophthalmicus in immunocompetent patients.[Cochrane Database Syst Rev. 2016]Review Valacyclovir versus acyclovir for the treatment of herpes zoster ophthalmicus in immunocompetent patients.Schuster AK, Harder BC, Schlichtenbrede FC, Jarczok MN, Tesarz J. Cochrane Database Syst Rev. 2016 Nov 14; 11(11):CD011503. Epub 2016 Nov 14.
- Review Management of herpes simplex and varicella-zoster virus infections.[West J Med. 1997]Review Management of herpes simplex and varicella-zoster virus infections.Erlich KS. West J Med. 1997 Mar; 166(3):211-5.
- Review Valacyclovir: a review of its antiviral activity, pharmacokinetic properties, and clinical efficacy.[Antiviral Res. 1995]Review Valacyclovir: a review of its antiviral activity, pharmacokinetic properties, and clinical efficacy.Beutner KR. Antiviral Res. 1995 Dec; 28(4):281-90.
- Valacyclovir - LiverToxValacyclovir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...