NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ganciclovir is a nucleoside analogue and antiviral agent used in therapy of cytomegalovirus infections. Valganciclovir is the valyl ester prodrug of ganciclovir which can be given orally. When given intravenously, ganciclovir can cause mild, transient and asymptomatic elevations in serum aminotransferase levels, but neither ganciclovir nor valganciclovir have been associated with clinically apparent liver injury.
Background
Ganciclovir (gan sye' kloe vir) is an acyclic guanosine nucleoside analogue structurally related to acyclovir which has antiviral activity against many herpes viruses, including herpes simplex 1 and 2, Epstein-Barr virus and varicella-zoster, but is used largely in the therapy of cytomegalovirus (CMV) infections. Ganciclovir is phosphorylated intracellularly by viral kinases and the resultant triphosphate competes with guanosine for incorporation into viral DNA, blocking viral DNA polymerase activity. Because of the requirement for activation by viral kinases, ganciclovir is not active in non-infected cells. Ganciclovir is not well absorbed orally and must be given parenterally to achieved adequate levels for maximal antiviral effect. Valganciclovir (val" gan sye' kloe vir) is the L-valyl ester prodrug of ganciclovir which is well absorbed orally and rapidly converted to ganciclovir by intracellular hydrolases. Ganciclovir and valganciclovir are indicated for therapy and prophylaxis of severe CMV infections. Ganciclovir was approved for use in the United States in 1994 and is available in a parenteral formulation in generic forms and under the brand name of Cytovene. The usual recommended regimen of therapy in adults for CMV retinitis or systemic infection is 5 mg/kg intravenously every 12 hours for 2 to 3 weeks, which can be followed by maintenance oral therapy using valganciclovir. Valganciclovir was approved for use in 2001 and is available in 450 mg tablets and as a powder for solution under the brand name of Valcyte. Valganciclovir is recommended in doses of 900 mg once or twice daily. Valganciclovir has replaced oral ganciclovir in therapy and prophylaxis of CMV infections. Side effects of ganciclovir and valganciclovir therapy include headache, dizziness, confusion, tremors, nausea, diarrhea, fever, renal dysfunction, bone marrow suppression and rash.
Hepatotoxicity
Intravenous administration of ganciclovir is associated with transient mild-to-moderate elevations in serum ALT levels in 2% of patients. These episodes have usually been asymptomatic and self-limited. CMV infection itself can cause liver enzyme elevations and may account for some abnormalities found during therapy. There is little evidence that either ganciclovir or valganciclovir can cause clinically apparent liver injury. Ganciclovir also has activity against HBV and HBV DNA levels decrease on treatment and can rebound when therapy is stopped, leading to an acute flare of hepatitis B, which can be symptomatic and severe.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Ganciclovir is metabolized intracellularly in viral infected cells, hepatic metabolism is minimal, and it is excreted largely unchanged by the kidneys, perhaps accounting for the absence or rarity of hepatic injury. The flare of hepatitis B that occurs when ganciclovir is stopped is probably caused by an immune reaction to the rapid return of HBV replication after therapy.
Drug Class: Antiviral Agents
Other Antiviral Agents for Herpes Virus Infections: Acyclovir, Cidofovir, Famciclovir, Foscarnet, Letermovir, Valacyclovir
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ganciclovir – Generic, Cytovene®
Valganciclovir – Generic, Valcyte®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ganciclovir | 82410-32-0 | C9-H13-N5-O4 |
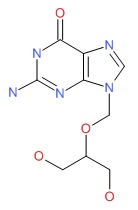 |
| Valganciclovir | 175865-60-8 | C14-H22-N6-O5 |
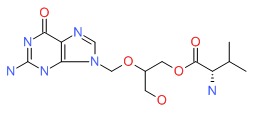 |
ANNOTATED BIBLIOGRAPHY
References updated: 06 March 2018
- Zimmerman HJ. Antiviral agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 621-3.(Expert review of antiviral agents and liver injury published in 1999; mentions that ganciclovir has not caused "overt hepatic injury").
- Núñez M. Hepatotoxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 505-18.(Review of hepatotoxicity of antiviral agents; ganciclovir and valganciclovir have been linked to serum enzyme elevations during therapy, and cases of clinically apparent liver injury have been reported to the sponsor).
- Acosta EP, Flexner C. Antiviral agents (nonretroviral). In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1593-1622.(Textbook of pharmacology and therapeutics).
- Styrt B, Freiman JP. Hepatotoxicity of antiviral agents. Gastroenterol Clin North Am 1995; 24: 839-52. [PubMed: 8749901](Review of liver toxicity of antiviral agents; intravenous administration of ganciclovir has been reported to cause serum aminotransferase elevations in 2% of patients).
- Herraiz M, Beraza N, Solano A, Snagro B, Montoya J, Qian C, Prieto J, et al. Liver failure caused by herpes simplex virus thymidine kinase plus ganciclovir therapy is associated with mitochondrial dysfunction and mitochondrial DNA depletion. Human Gene Ther 2003; 14: 463-72. [PubMed: 12691611](In a rat model, the adenoviral transfer of thymidine kinase followed by ganciclovir leads to acute hepatocellular injury with evidence of mitochondrial injury, lactic acid elevations and morphologic changes; this form of toxicity is only a concern for gene therapy studies in which there may be gene transfer of the viral kinase to normal hepatocytes).
- Reusser P. Oral valganciclovir: a new option for treatment of cytomegalovirus infection and disease in immunocompromised hosts. Expert Opin Investig Drugs 2001; 10: 1745-53. [PubMed: 11772283](Review of valganciclovir, an oral prodrug of ganciclovir, which is active against CMV infection and disease; no mention of hepatotoxicity or ALT elevations with therapy).
- Shea BF, Hoffman S, Sesin GP, Hammer SM. Ganciclovir hepatotoxicity. Pharmacotherapy 1987; 7: 223-6. [PubMed: 2832836](33 year old man with HIV infection, CMV retinitis and mycobacterium avium intracellulare [MAC] infections developed increases in preexisting elevated enzyme levels without bilirubin elevations during two courses of ganciclovir [ALT 133 rising to ~500 U/L, AlkP 181 rising to 1014 U/L], peaking several weeks after stopping and then decreasing; liver biopsy showed MAC and no evidence of hepatitis).
- Hochster H, Dieterich D, Bozzette S, Reichman RC, Connor JD, Liebes L, Sonke RL, et al. Toxicity of combined ganciclovir and zidovudine for cytomegalovirus disease associated with AIDS. An AIDS Clinical Trials Group Study. Ann Intern Med 1990; 113: 111-7. [PubMed: 2163228](40 patients with HIV and CMV infection were treated with zidovudine and intravenous ganciclovir; anemia and neutropenia were common; 1 patient developed hepatitis thought to be zidovudine-related, resolving on stopping and recurred on restarting zidovudine [timing and details not given]).
- Figge HL, Bailie GR, Briceland LL, Kowalsky SF. Possible ganciclovir-induced hepatotoxicity in patients with AIDS. Clin Pharm 1992; 11: 432-4. [PubMed: 1316254](Retrospective review of 14 courses of ganciclovir in 11 patients with HIV infection identified 5 with associated elevations in AST [29-36 rising to 55-110 U/L] or Alk P [61 rising to 360 U/L], but all were receiving other potentially hepatotoxic agents and no patient was reported to develop hepatitis or jaundice).
- Gish RG, Lau JY, Brooks L, Fang WS, Steady SL, Imperial JC, Garcia-Kennedy R, et al. Ganciclovir treatment of hepatitis B virus infection in liver transplant recipients. Hepatology 1996; 23: 1-7. [PubMed: 8550028](9 patients with recurrent hepatitis B after liver transplant were treated with ganciclovir for 3-10 months; serum HBV DNA and ALT levels decreased on therapy and flare occurred in 3 when ganciclovir was stopped, one becoming jaundiced and developing hepatic decompensation).
- Noormohamed SE. Potential of ganciclovir to induce hepatotoxicity in patients with HIV disease. J Infect Dis Pharmacother 1997; 2: 95-101. Not in PubMed.
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008; 8 were attributed to antiviral agents including one attibuted to valacyclovir, but none were attributed to ganciclovir or valganciclovir).
- Witzke O, Hauser IA, Bartels M, Wolf G, Wolters H, Nitschke M; VIPP Study Group. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation 2012; 93: 61-8. [PubMed: 22094954](Controlled trial of prophylaxis vs preemptive therapy of CMV infection in 296 renal allograft recipients; overall tolerability was good and no mention of ALT elevations or hepatotoxicity).
- Antiviral drugs. Treat Guidel Med Lett 2013; 11 (127): 19-30. [PubMed: 23459414](Review of safety and efficacy of ganciclovir and valganciclovir treatment and prophylaxis against CMV infection; mentions that adverse effects include "abnormal liver function").
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, two cases were attributed to valacyclovir and one to acyclovir, but none to ganciclovir or valganciclovir).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Pharmacokinetics of valganciclovir and ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-seropositive volunteers.[Clin Pharmacokinet. 1999]Pharmacokinetics of valganciclovir and ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-seropositive volunteers.Brown F, Banken L, Saywell K, Arum I. Clin Pharmacokinet. 1999 Aug; 37(2):167-76.
- Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2.[J Pharm Sci. 2000]Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2.Sugawara M, Huang W, Fei YJ, Leibach FH, Ganapathy V, Ganapathy ME. J Pharm Sci. 2000 Jun; 89(6):781-9.
- Review Valganciclovir in adult solid organ transplant recipients: pharmacokinetic and pharmacodynamic characteristics and clinical interpretation of plasma concentration measurements.[Clin Pharmacokinet. 2009]Review Valganciclovir in adult solid organ transplant recipients: pharmacokinetic and pharmacodynamic characteristics and clinical interpretation of plasma concentration measurements.Perrottet N, Decosterd LA, Meylan P, Pascual M, Biollaz J, Buclin T. Clin Pharmacokinet. 2009; 48(6):399-418.
- Review Valganciclovir: oral prevention and treatment of cytomegalovirus in the immunocompromised host.[Expert Opin Pharmacother. 2004]Review Valganciclovir: oral prevention and treatment of cytomegalovirus in the immunocompromised host.Freeman RB. Expert Opin Pharmacother. 2004 Sep; 5(9):2007-16.
- A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis.[N Engl J Med. 2002]A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis.Martin DF, Sierra-Madero J, Walmsley S, Wolitz RA, Macey K, Georgiou P, Robinson CA, Stempien MJ, Valganciclovir Study Group. N Engl J Med. 2002 Apr 11; 346(15):1119-26.
- Ganciclovir - LiverToxGanciclovir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...