NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tolvaptan is a vasopressin 2 receptor antagonist which is used for short term treatment of severe hyponatremia in patients with heart failure, cirrhosis or syndrome of inappropriate secretion of antidiuretic hormone (SIADH). It has been used experimentally to prevention progression of disease in autosomal dominant polycystic kidney disease (ADPKD). Tolvaptan recently has been implicated in causing serum aminotransferase elevations as well as clinically apparent acute liver injury during long term use.
Background
Tolvaptan (tol vap' tan) is a vasopressin 2 receptor antagonist (vaptan) that is used for treatment of hyponatremia caused by elevated levels of arginine vasopressin (also known as antidiuretic hormone: ADH), commonly found in patients with inappropriate ADH syndrome (SIADH) or with fluid overload from heart failure or cirrhosis. Vasopressin acts on type 2 receptors in the distal renal tubules causing reabsorption of free water, without electrolytes. Inappropriate secretion of vasopressin (as occurs in some paraneoplastic syndromes) is associated with retention of water and dilutional hyponatremia that can be symptomatic and even fatal. In controlled clinical trials, tolvaptan given for 28 days resulted in an increase in serum sodium and diuresis in patients with hypervolemic hyponatremia, in patients with cirrhosis and heart failure, and euvolemic hyponatremia in patients with SIADH. Tolvaptan was approved for use in the United States in 2009 and current indications are for short term therapy of patients with hypervolemic or euvolemic hyponatremia due to SIADH, congestive heart failure or cirrhosis. Tolvaptan has also been shown to prevent progression of disease in patients with autosomal dominant polycystic kidney disease (ADPKD), and was approved for this use in the United States in 2019. For therapy of hyponatremia, tolvaptan is available in tablets of 15 and 30 mg under the brand name Samsca. The recommended dose is 15 mg initially, titrating up to a maximum of 60 mg once daily, but limiting therapy to 30 days. For therapy of autosomal dominant polycystic kidney disease, tolvaptan is available in tablets of 15 and 30 mg under the brand name Jynarque and the recommended dose is 60 mg initially (given in two divided doses) and titrating carefully up to a maximum of 120 mg daily. Common side effects include excessive thirst, dry mouth and urinary frequency. Rare, but more serious side effects include hypernatremia and osmotic demyelination injury and acute liver injury.
Hepatotoxicity
In prelicensure clinical trials, tolvaptan was not implicated in causing serum enzyme elevations or clinically apparent liver injury. However, instances of worsening of hepatic failure and complications of portal hypertension were reported in a small proportion of patients with cirrhosis treated with tolvaptan. These complications included variceal hemorrhage, hepatic encephalopathy and worsening of jaundice. In many trials, however, the frequency of these complications was not significantly greater than in placebo treated controls. More recently, in large registration trials of long term therapy in patients with ADPKD, serum aminotransferase elevations occurred in 4% to 5% of patients on tolvaptan, compared to only 1% of controls. Furthermore, clinically apparent liver injury occurred in approximately 0.1% of treated patients. The time to onset of illness ranged from 3 to 9 months (Case 1), but occasionally arose during long term therapy (Case 2). The clinical presentation was with the insidious development of fatigue, nausea and abdominal pain followed by dark urine, jaundice and pruritus. The pattern of serum enzyme elevations was typically hepatocellular or mixed, and liver biopsy showed an acute hepatitis with mild cholestasis. All patients recovered after stopping therapy, generally within 1 to 3 months of stopping therapy without evidence of residual injury. Immunoallergic features and autoantibodies were not found. Rapid recurrence on rechallenge was demonstrated in several patients with marked serum enzyme elevations during therapy, but patients with jaundice were not reexposed. The frequency of clinically apparent liver injury during therapy was one reason for the delay of formal approval of long term tolvaptan therapy for ADPKD. Since its approval and more wide-spread use, occasion reports of clinically apparent liver injury have continued to appear, at least one of which led to liver transplantation. Interestingly, most instances of liver injury have been reported with its use in autosomal dominant polycystic kidney disease rather than hyponatremia. Reasons for this are probably the duration of therapy, but also may relate to the slightly higher doses used to decrease progress in polycystic kidney disease.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
Tolvaptan is metabolized by the microsomal P450 drug metabolizing enzyme CYP 3A4 liver injury from tolvaptan may be due to activation of a toxic intermediate. Inhibitors of CYP 3A4 (such as ketoconazole) can raise levels of tolvaptan and should be avoided.
Outcome and Management
The hepatic injury caused by tolvaptan is usually reversible with stopping the medication. Tolvaptan has not been linked to cases of acute liver failure, chronic hepatitis, prolonged cholestasis or vanishing bile duct syndrome. Rechallenge usually causes recurrence and should be avoided. There is no information on possible cross sensitivity to liver injury among various vasopressin 2 receptor antagonists, such as satavaptan, lixivaptan or conivaptan.
Drug Class: Diuretics, Vasopressin Antagonists
CASE REPORTS
Case 1. Acute hepatitis with jaundice attributed to tolvaptan therapy.(1,2)
A 45 year old woman with autosomal dominant polycystic kidney disease (ADPKD) developed mild symptoms of fatigue, abdominal pain, anorexia and nausea approximately 5 months after starting tolvaptan as a part of a controlled trial of this agent in ADPKD. She had no previous history of liver disease, alcohol use, or risk factors for viral hepatitis or drug allergies. Her liver tests had been normal before treatment and again 4 months after starting tolvaptan. Her other medical conditions included renal insufficiency, recurrent urinary tract infections, hypertension and osteoarthritis. Other medications included atenolol, impidapril and olmesartan, all of which she had taken chronically. Tolvaptan was continued and she was monitored more frequently. Tests for viral hepatitis and other causes of liver disease were said to be negative. Her symptoms improved for a few days, but then worsened as did serum enzyme abnormalities and serum bilirubin (Table). Tolvaptan was stopped approximately 6 weeks after onset of symptoms. Nevertheless, serum bilirubin levels continued to rise and peaked at 7.6 mg/dL 11 days after stopping tolvaptan. Subsequently, symptoms resolved and serum enzymes fell into the normal range within the next two months.
Key Points
| Medication: | Tolvaptan (120 mg daily) |
|---|---|
| Pattern: | Hepatocellular (R=16.2) |
| Severity: | Moderate (hospitalized) |
| Latency: | 4 months |
| Recovery: | 2 months after stopping |
| Other medications: | Atenolol, impidapril, olmesartan |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | GGT (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|---|
| Pre | Pre | 16 | 14 | 0.4 | Tolvaptan started | |
| 123 days | Pre | 46 | 196 | 31 | 0.5 | |
| 166 days | Pre | 570 | 358 | 274 | 0.5 | Symptomatic |
| 177days | Pre | 332 | 262 | 246 | 0.5 | |
| 190 days | Pre | 159 | 215 | 199 | 0.6 | |
| 202 days | 0 | 882 | 338 | 323 | 1.4 | Tolvaptan stopped |
| 207 days | 5 days | 809 | 316 | 244 | 4.1 | |
| 213 days | 11 days | 598 | 325 | 206 | 7.6 | |
| 222 days | 22 days | 200 | 240 | 189 | 2.0 | |
| 232 days | 32 days | 57 | 170 | 124 | 1.8 | |
| 249 days | 49 days | 30 | 62 | 1.1 | ||
| Normal Values | <35 | <350 | <50 | <1.2 | ||
Comment
This patient developed symptoms and serum enzyme elevations 4 months after starting tolvaptan. The medication was continued and, after improving temporarily, she developed further symptoms and jaundice. No other cause of the abnormalities was found and all liver tests fell into the normal range within two months of stopping.
Case 2. Acute hepatitis with jaundice attributed to tolvaptan therapy.(2,3)
A 44 year old woman with autosomal dominant polycystic kidney disease (ADPKD) participating in an experimental study of tolvaptan was found to have serum enzyme elevations at a routine 3 month study visit. She reported having mild and transient nausea and abdominal pain during the previous several weeks, but denied jaundice or dark urine. She had no previous history of liver disease or drug allergies. Her liver tests had been repeatedly normal in the past including during a three year period of taking placebo as a participant in a randomized controlled trial of tolvaptan. Tests were also normal just before starting open-label tolvaptan therapy (Table). She did not drink alcohol and had no risk factors for viral hepatitis. Her other medical conditions included renal insufficiency, recurrent urinary tract infections, hypertension and osteoarthritis. Other medications included perindopril, an antihypertensive, angiotensin converting enzyme (ACE) inhibitor available in Europe. Tolvaptan was stopped promptly, and she was admitted for evaluation and monitoring. During the ensuing weeks she developed more persistent symptoms of fatigue, nausea and anorexia followed by dark urine and jaundice. Tests for hepatitis A, B, C and E and mononucleosis were negative as were antinuclear and smooth muscle antibodies. Abdominal ultrasound and magnetic resonance imaging demonstrated multiple kidney and hepatic cysts, but no evidence of biliary obstruction or hepatic masses. A liver biopsy showed a cholestatic hepatitis with focal necrosis consistent with drug induced liver injury. In follow up, her symptoms resolved and liver tests were improved when she was seen 3 months after initial onset. During long term follow up, however, she continued to have mild elevations in serum aminotransferase levels (< twice ULN).
Key Points
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | GGT (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | Pre | 12 | 16 | 0.4 | Tolvaptan started |
| 89 days | 0 | 1243 | 122 | 0.8 | Tolvaptan stopped |
| 98 days | 8 days | 1098 | 190 | 1.2 | Symptomatic |
| 108 days | 18 days | 1742 | 208 | 9.6 | |
| 4 months | 1 month | 746 | 164 | 10.2 | Liver biopsy |
| 6 months | 3 months | 59 | 63 | 0.6 | |
| 7 months | 4 months | 59 | 54 | 0.6 | |
| Normal Values | <35 | <50 | <1.2 | ||
Comment
This patient developed a moderately severe acute hepatitis 90 days after starting tolvaptan in an experimental, open-label, rollover trial of this agent given long term in patients with symptomatic autosomal dominant polycystic kidney disease. The injury was detected during a routine visit and tolvaptan was promptly stopped. However, she developed symptoms and jaundice over the ensuing weeks with serum bilirubin rising to a peak of 10.2 mg/dL. A liver biopsy showed a cholestatic hepatitis without extensive necrosis. She was symptomatic for several weeks but eventually recovered, although she continued to have mild serum ALT and AST elevations in subsequent follow up. This was one of three cases of acute liver injury with jaundice that arose during the clinical development of tolvaptan as therapy for ADPKD.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tolvaptan – Jynarque®, Samsca®
DRUG CLASS
Diuretics, Vasopressin Antagonists
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Tolvaptan | 150683-30-0 | C26-H25-Cl-N2-O3 |
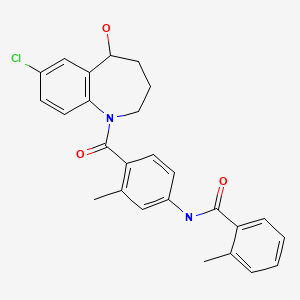
|
CITED REFERENCES
- 1.
- Clinical review. Tolvaptan; NDA 204441: Case 04251-731-2738.
- 2.
- Watkins PB, Lewis JH, Kaplowitz N, Alpers DH, Blais JD, Smotzer DM, Krasa H, et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015;38:1103–13. [PMC free article: PMC4608984] [PubMed: 26188764]
- 3.
- Clinical review. Tolvaptan; NDA 204441: Case 08271-468-4301.
ANNOTATED BIBLIOGRAPHY
References updated: 02 September 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999 before the availability of tolvaptan or conivaptan).
- Jackson EK. Drugs affecting renal excretory function. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 466-9.(Textbook of pharmacology and therapeutics; arginine vasopressin 2 receptors antagonists are aquaretics and nonpeptide inhibitors include conivaptan, tolvaptan, lixivaptan and mozavaptan).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2018/204441Orig1s000MedR.pdf [Medical Review: 230-257; 259-267] (FDA analysis of hepatotoxicity identified in the registration trials of tolvaptan for polycystic liver disease). - Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–112. [PubMed: 17105757](Summary of two controlled trials of 30 day courses of tolvaptan in 448 patients with hyponatremia due to SIADH, heart failure or cirrhosis found significant rise in serum sodium [6.2 vs 1.8 mmol/L], but few side effects except for thirst and dry mouth; with no reports of liver adverse events in tolvaptan treated patients).
- Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–31. [PubMed: 17384437](Controlled trial of tolvaptan [15 mg daily] vs placebo for 60 days in 4133 patients hospitalized for worsening heart failure found no effect of treatment on long term mortality; discussion and lists of side effects did not include ALT or AST elevations or clinically apparent liver injury).
- Ginès P. Vaptans: a promising therapy in the management of advanced cirrhosis. J Hepatol. 2007;46:1150–2. [PubMed: 17445935](Discussion of publication by Schrier [2006] mentioning the need for separate analysis and future studies of patients with hyponatremia due to cirrhosis as efficacy and safety may be different in this subgroup).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to tolvaptan or conivaptan).
- Tolvaptan (Samsca) for hyponatremia. Med Lett Drugs Ther. 2009;51(1326):95–6. [PubMed: 20224525](Concise review of the mechanism of action, efficacy and safety of tolvaptan shortly after its approval in the US does not mention ALT elevations or hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to tolvaptan or conivaptan).
- Nemerovski C, Hutchinson DJ. Treatment of hypervolemic or euvolemic hyponatremia associated with heart failure, cirrhosis, or the syndrome of inappropriate antidiuretic hormone with tolvaptan: a clinical review. Clin Ther. 2010;32:1015–32. [PubMed: 20637957](Review of the mechanism of action, efficacy and safety of tolvaptan; no mention of hepatotoxicity).
- Okita K, Sakaida I, Okada M, Kaneko A, Chayama K, Kato M, Sata M, et al. A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose-response of tolvaptan in patients with decompensated liver cirrhosis. J Gastroenterol. 2010;45:979–87. [PubMed: 20387081](Among 18 patients with cirrhosis and intractable ascites in whom tolvaptan was added to stable doses of diuretics, esophageal varices arose in 2 and encephalopathy in 1 patient; no mention of changes in routine liver test results).
- Boyer TD. Tolvaptan and hyponatremia in a patient with cirrhosis. Hepatology. 2010;51:699–702. [PubMed: 20101750](Discussion of the pathophysiology of fluid overload and hyponatremia in cirrhosis [with clinical case example] and possible role and safety of tolvaptan; in controlled trials, gastrointestinal bleeding occurred in 10% of tolvaptan- vs 2% of placebo-treated patients).
- Matsuzaki M, Hori M, Izumi T, Asanoi H, Tsutamoto T. Tolvaptan Investigators. Effects of tolvaptan on volume overload in Japanese patients with heart failure: results of a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Cardiovasc Drugs Ther. 2011;25 Suppl 1:S19–31. [PubMed: 22120091](In a controlled trial of tolvaptan [15, 30 or 45 mg once daily] vs placebo for 7 days in 117 patients with heart failure and fluid overload, tolvaptan was not associated with any major clinical concerns and was "clinically tolerable at all doses tested").
- Matsuzaki M, Hori M, Izumi T, Fukunami M. Tolvaptan Investigators. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study(QUEST study). Cardiovasc Drugs Ther. 2011;25 Suppl 1:S33–45. [PubMed: 22120092](Controlled trial of tolvaptan [15 mg daily] vs placebo for 7 days in 110 patients with heart failure and fluid overload; side effects included thirst, constipation, urinary frequency and fatigue; ALT elevations and clinically apparent liver injury were not mentioned).
- Fukunami M, Matsuzaki M, Hori M, Izumi T. Tolvaptan Investigators. Efficacy and safety of tolvaptan in heart failure patients with sustained volume overload despite the use of conventional diuretics: a phase III open-label study. Cardiovasc Drugs Ther. 2011;25 Suppl 1:S47–56. [PubMed: 22120093](Among 51 patients with congestive heart failure and fluid overload treated with tolvaptan for 7-14 days, ALT levels were increased in 6%, but no patient had a serious liver related adverse event).
- Cabello Muriel A, Marín Pozo JF, Alcalá Sanz A, Carrillo Ortiz D. Hepatic alteration after treatment using tolvaptan. Farm Hosp. 2011;35:94–6. [PubMed: 20864371](75 year old man with lung cancer and symptomatic hyponatremia [sodium 120-128 mEq/L] due to SIADH developed liver test abnormalities 24 days after starting tolvaptan [ALT 378 U/L, GGT 3585 U/L, bilirubin and Alk P not given], which improved on stopping, but with limited follow up, as he died soon thereafter).
- Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, Horie S, et al. TEMPO Formula and 156-05-002 Study Investigators. Tolvaptan in autosomal dominant polycystic kidney disease: three years' experience. Clin J Am Soc Nephrol. 2011;6:2499–507. [PMC free article: PMC3359559] [PubMed: 21903984](Analysis of two 3-year studies of tolvaptan in 63 patients with polycystic kidney disease; ALT elevations occurred in 2 patients [3.2%]; no details given).
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, et al. TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–18. [PMC free article: PMC3760207] [PubMed: 23121377](Controlled trial of tolvaptan [up to 120 mg daily] vs placebo for 3 years in 1445 adults with polycystic kidney disease reported less increase in kidney cyst size and less decrease in renal function with tolvaptan; ALT elevations >2.5 times ULN occurred in 4.9% on tolvaptan vs 1.2% on placebo and 2 patients developed ALT elevations with jaundice, but no patient died and "the abnormalities either resolved during treatment or returned towards baseline values with drug interruption or withdrawal").
- Wüthrich RP, Mei C. Aquaretic treatment in polycystic kidney disease. N Engl J Med. 2012;367:2440–2. [PubMed: 23121376](Editorial in response to Torres [2012]).
- Dahl E, Gluud LL, Kimer N, Krag A. Meta-analysis: the safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther. 2012;36:619–26. [PubMed: 22908905](Systematic review of vaptans identified 12 controlled trials in a total of 2266 patients with cirrhosis and ascites; therapy had no effect on mortality or rates of variceal hemorrhage; no mention of ALT elevations or other liver complications).
- Gaglio P, Marfo K, Chiodo J 3rd. Hyponatremia in cirrhosis and end-stage liver disease: treatment with the vasopressin V.-receptor antagonist tolvaptan. Dig Dis Sci. 2012;57:2774–85. [PMC free article: PMC3472061] [PubMed: 22732834](Review of frequency, clinical features and complications of hyponatremia in cirrhosis and the possible role of tolvaptan in its management).
- Ambrosy AP, Vaduganathan M, Huffman MD, Khan S, Kwasny MJ, Fought AJ, Maggioni AP, et al. EVEREST trial investigators. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail. 2012;14:302–11. [PubMed: 22357577](Analysis of 2061 patients with heart failure enrolled in placebo arm of tolvaptan trials found that liver test abnormalities were frequent with ALT elevations in 21%, Alk P in 23% and bilirubin in 26%, but most elevations were mild and improved with therapy of heart failure).
- Sakaida I, Yanase M, Kobayashi Y, Yasutake T, Okada M, Okita K. The pharmacokinetics and pharmacodynamics of tolvaptan in patients with liver cirrhosis with insufficient response to conventional diuretics: a multicentre, double-blind, parallel-group, phase III study. J Int Med Res. 2012;40:2381–93. [PubMed: 23321196](Among 40 patients with cirrhosis and intractable ascites treated with tolvaptan [3.75 or 7.5 mg daily for 7 days], adverse events included hepatic encephalopathy in 4 [10%], but no specifics given).
- Cho C, Logan JL, Lien YH. Massive aquaresis after tolvaptan administration and albumin infusion in a patient with alcoholic cirrhosis. Am J Med. 2012;125(1):e5–6. [PubMed: 22075044](40 year old man with cirrhosis and hypervolemic hyponatremia had marked transient diuresis [from <50 to 800 mL/hr] after a single dose of tolvaptan, paracentesis and albumin infusions).
- Cárdenas A, Ginès P, Marotta P, Czerwiec F, Oyuang J, Guevara M, Afdhal NH. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol. 2012;56:571–8. [PubMed: 22027579](Subanalysis of trials reported by Schrier [2006] limited to patients with cirrhosis treated with tolvaptan [n=63] vs placebo [n=57] for 30 days; serious liver related adverse events occurred in 3 and deaths from liver disease in 2 tolvaptan treated subjects; gastrointestinal bleeding occurred in 6 [10%] tolvaptan- vs 1 [2%] placebo-treated patients).
- Sakaida I, Kawazoe S, Kajimura K, Saito T, Okuse C, Takaguchi K, Okada M, et al. ASCITES-DOUBLEBLIND Study Group. Tolvaptan for improvement of hepatic edema: A phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44(1):73–82. [PubMed: 23551935](Controlled trial of 7 days of tolvaptan vs placebo in 162 patients with cirrhosis and ascites found slight increase in serum sodium [1.2 vs -0.7] and decrease in body weight [-1.9 vs-0.4 kg] and ascites with tolvaptan therapy; "No marked abnormalities were clinically observed in clinical laboratory tests").
- Yu C, Sharma N, Saab S. Hyponatremia: clinical associations, prognosis, and treatment in cirrhosis. Exp Clin Transplant. 2013;11:3–11. [PubMed: 23387536](Review of the management of hyponatremia in cirrhosis states that the effect of vaptans is dose dependent, starts 1-2 hours after administration, lasts for 4-12 hours, and is lost within a week of withdrawal, suggesting that continuous or long term therapy is needed).
- Abhyankar A, Robson SC, Tapper EB, Bonder A. Letter in response to the recently published review: hyponatremia in cirrhosis and end-stage liver disease-treatment with the vasopressin v2-receptor antagonist tolvaptan. Dig Dis Sci. 2013;58:889–90. [PubMed: 23371016](Summary of experience in treating 9 patients with cirrhosis and hyponatremia using oral tolvaptan for periods ranging from 9 days to 2 years; the major "patient issues" were intense thirst and high cost; no mention of liver related side effects).
- Yakushijin K, Yamamoto K, Kurata K, Miyata Y, Kakiuchi S, Tomioka H, Kawamori-Iwamoto Y, et al. Tolvaptan as an alternative treatment for refractory fluid retention associated with sinusoidal obstruction syndrome after allogeneic stem cell transplantation. Int J Hematol. 2013;97:284–6. [PubMed: 23297121](42 year old man with lymphoma developed sinusoidal obstruction syndrome 3 weeks after allogenic hematopoietic cell transplantation [with myeloablation with busulfan and cyclophosphamide] and was given two days of tolvaptan for ascites and fluid overload followed by rise of serum sodium to 159 mEq/L; patient ultimately died of hepatic and multiorgan failure).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to tolvaptan).
- Watkins PB, Lewis JH, Kaplowitz N, Alpers DH, Blais JD, Smotzer DM, Krasa H, et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015;38:1103–13. [PMC free article: PMC4608984] [PubMed: 26188764](Among 1445 patients with autosomal dominant polycystic kidney disease treated with tolvaptan or placebo, 4.4% receiving tolvaptan vs 1% receiving placebo developed ALT elevations above 3 times ULN, between 3-18 months after starting and resolving in all; 2 developing jaundice and symptoms [as did another who was crossed over from placebo to tolvaptan: cases 1 and 2]; similar episodes were not seen in the 2250 subjects treated with tolvaptan for hyponatremia; Cases 1 and 2).
- Mosedale M, Kim Y, Brock WJ, Roth SE, Wiltshire T, Eaddy JS, Keele GR, et al. Editor's Highlight: Candidate risk factors and mechanisms for tolvaptan-Induced liver injury are identified using a collaborative cross approach. Toxicol Sci. 2017;156:438–54. [PMC free article: PMC6075566] [PubMed: 28115652](Screening of mouse strains that develop ALT elevations in response to tolvaptan found that genes of bile acid homeostasis as well as oxidative stress and immune response were activated by exposure to the drug).
- Muto S, Okada T, Yasuda M, Tsubouchi H, Nakajima K, Horie S. Long-term safety profile of tolvaptan in autosomal dominant polycystic kidney disease patients: TEMPO Extension Japan Trial. Drug Healthc Patient Saf. 2017;9:93–104. [PMC free article: PMC5661830] [PubMed: 29123425](Among 135 Japanese patients with autosomal dominant polycystic kidney disease enrolled in an open label extension trial of tolvaptan for up to 3 years, 5.9% developed ALT elevations above 3 times ULN, but none had concurrent symptoms or jaundice and all resolved spontaneously or with dose interruption).
- Makabe S, Mochizuki T, Mitobe M, Aoyama Y, Kataoka H, Tsuchiya K, Nitta K. Elevation of the serum liver enzyme levels during tolvaptan treatment in patients with autosomal dominant polycystic kidney disease (ADPKD). Clin Exp Nephrol. 2018;22:1079–87. [PubMed: 29508162](Among 63 Japanese patients with autosomal dominant polycystic kidney disease treated with tolvaptan, 7 developed ALT elevations between months 2 and 8 [ALT 37 to 77 U/L] and tolvaptan was stopped, but was subsequently restarted in 6 whose ALT levels rapidly improved of whom only one had any recurrence of enzyme elevations; in one subject ALT levels increased after topping tolvaptan to a peak of 980 U/L and drug was not restarted; none developed bilirubin elevations or symptoms).
- Khan MY, Rawala MS, Siddiqui M, Abid W, Aslam A. Tolvaptan-induced liver injury: who is at risk? a case report and literature review. Cureus. 2019;11(6):e4842. [PMC free article: PMC6684126] [PubMed: 31410325](86 year old woman with inappropriate antidiuretic hormone syndrome and hyponatremia [118 mmoL/L] was found to have liver test abnormalities the day after starting tolvaptan [bilirubin 2.8 mg/dL, ALT 193 U/L, Alk P 424 U/L], which rose over the next several days despite stopping tolvaptan and were still elevated when last seen 7 days later [bilirubin 5.5 mg/dL, ALT 164 U/L, Alk P 815 U/L] with no further information on the course of illness).
- Endo M, Katayama K, Matsuo H, Horiike S, Nomura S, Hayashi A, Ishikawa E, et al. Role of liver transplantation in tolvaptan-associated acute liver failure. Kidney Int Rep. 2019;4:1653–7. [PMC free article: PMC6933473] [PubMed: 31891010](36 year old Japanese woman with autosomal dominant polycystic kidney disease developed abnormal ALT levels 5 months after starting tolvaptan that continued to rise despite dose modification and discontinuation [bilirubin 5.2 mg/dL, ALT 426 U/L, Alk P 305 U/L, INR 2.8], with progressive hepatic failure leading to liver transplantation).
- Pellegrino AM, Annicchiarico Petruzzelli L, Riccio E, Pisani A. Idiosyncratic hepatic toxicity in autosomal dominant polycystic kidney disease (ADPKD) patient in combined treatment with tolvaptan and amoxicillin/clavulanic acid: a case report. BMC Nephrol. 2019;20:426. [PMC free article: PMC6873754] [PubMed: 31752750](41 year old woman with autosomal dominant polycystic kidney disease developed ALT elevations at week 12 of therapy with tolvaptan and 5 weeks after receiving a course of amoxicillin-clavulanate, which continued to worsen to an ALT peak of 808 U/L, eventually falling into the normal range by week 24; no mention of bilirubin or Alk P levels).
- Sakaida I, Terai S, Kurosaki M, Okada M, Hirano T, Fukuta Y. Real-world effectiveness and safety of tolvaptan in liver cirrhosis patients with hepatic edema: results from a post-marketing surveillance study (START study). J Gastroenterol. 2020;55:800–10. [PMC free article: PMC7376514] [PubMed: 32388692](In postmarketing studies on the safety of tolvaptan in 1,111 Japanese patients with cirrhosis who were treated with a mean daily dose of 6 mg for an average of 82 days, side effects included thirst [6.6%], hepatic encephalopathy [2.3%], dehydration [1.5%] and hypernatremia [1.5%] and there were 157 deaths, 15 of which were considered possibly related to tolvaptan).
- Raina R, Chakraborty R, DeCoy ME, Kline T. Autosomal-dominant polycystic kidney disease: tolvaptan use in adolescents and young adults with rapid progression. Pediatr Res. 2020 May 11; In Press. [PubMed: 32392574](Among 52 young adults [ages 18 to 24 years] enrolled in the TEMPO 3:4 Trial which compared tolvaptan to placebo in patients with autosomal dominant polycystic kidney disease, clinical response rates were similar in younger vs older subjects, but none of the young subjects developed serum ALT elevations during therapy compare to 4% of the older adults).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Treatment of hypervolemic or euvolemic hyponatremia associated with heart failure, cirrhosis, or the syndrome of inappropriate antidiuretic hormone with tolvaptan: a clinical review.[Clin Ther. 2010]Review Treatment of hypervolemic or euvolemic hyponatremia associated with heart failure, cirrhosis, or the syndrome of inappropriate antidiuretic hormone with tolvaptan: a clinical review.Nemerovski C, Hutchinson DJ. Clin Ther. 2010 Jun; 32(6):1015-32.
- Review An update on tolvaptan for autosomal dominant polycystic kidney disease.[Drugs Today (Barc). 2018]Review An update on tolvaptan for autosomal dominant polycystic kidney disease.Poch E, Rodas L, Blasco M, Molina A, Quintana L. Drugs Today (Barc). 2018 Sep; 54(9):519-533.
- [Tolvaptan, a vasopressin V(2) receptor antagonist, is the world's first approved drug for treatment of autosomal dominant polycystic kidney disease (ADPKD)].[Nihon Yakurigaku Zasshi. 2022][Tolvaptan, a vasopressin V(2) receptor antagonist, is the world's first approved drug for treatment of autosomal dominant polycystic kidney disease (ADPKD)].Yamada Y, Fujiki H, Mizuguchi H, Takeshita Y, Hattori K, Ohmoto K, Aihara M, Nagano K, Isakari Y, Yamamoto M, et al. Nihon Yakurigaku Zasshi. 2022; 157(4):254-260.
- Clinical Pattern of Tolvaptan-Associated Liver Injury in Subjects with Autosomal Dominant Polycystic Kidney Disease: Analysis of Clinical Trials Database.[Drug Saf. 2015]Clinical Pattern of Tolvaptan-Associated Liver Injury in Subjects with Autosomal Dominant Polycystic Kidney Disease: Analysis of Clinical Trials Database.Watkins PB, Lewis JH, Kaplowitz N, Alpers DH, Blais JD, Smotzer DM, Krasa H, Ouyang J, Torres VE, Czerwiec FS, et al. Drug Saf. 2015 Nov; 38(11):1103-13.
- Rapidity of Correction of Hyponatremia Due to Syndrome of Inappropriate Secretion of Antidiuretic Hormone Following Tolvaptan.[Am J Kidney Dis. 2018]Rapidity of Correction of Hyponatremia Due to Syndrome of Inappropriate Secretion of Antidiuretic Hormone Following Tolvaptan.Morris JH, Bohm NM, Nemecek BD, Crawford R, Kelley D, Bhasin B, Nietert PJ, Velez JCQ. Am J Kidney Dis. 2018 Jun; 71(6):772-782. Epub 2018 Feb 23.
- Tolvaptan - LiverToxTolvaptan - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...