NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tolterodine is an anticholinergic agent used to treat urinary incontinence and overactive bladder syndrome. Tolterodine therapy has not been associated liver enzyme elevations while only a single case report has been published of clinical apparent acute liver injury attributed to its use.
Background
Tolterodine (tol ter' oh deen) is a synthetic anticholinergic and antispasmotic agent that inhibits muscarinic actions of acetylcholine on autonomic nerve endings, decreasing secretions and inhibiting gastrointestinal and bladder motility. Tolterodine increases bladder capacity and decreases bladder contractions and the frequency and urgency of urination. Tolterodine was approved for use in the United States in 1998 and indications include overactive bladder syndrome symptomatic with urinary frequency, urgency, or incontinence. Tolterodine is available in tablets of 1 and 2 mg and as extended release capsules of 2 and 4 mg in generic forms and under the brand name Detrol. The recommended adult oral dose is 2 to 4 mg daily. Common side effects are those of parasympathetic stimulation and include dryness of the mouth and eyes, decreased sweating, headache, visual blurring, constipation, urinary retention, restlessness, confusion and hallucinations. Rare but potentially severe adverse reactions include acute narrow angle glaucoma, acute urinary retention, gastric retention and stasis, neurologic symptoms and worsening of neurologic diseases, prolongation of the QTc interval, and severe hypersensitivity reactions.
Hepatotoxicity
In multiple randomized controlled trials of tolterodine in patients with overactive bladder syndrome, serum aminotransferase and alkaline phosphatase elevations were not common, arising in less than 1% of treated subjects and in a similar proportion of placebo recipients. In these clinical trials there were no reports of clinically apparent liver injury or jaundice. Since the approval of tolterodine in 1998 and its widescale use (with more than 1 million prescriptions filled yearly in the United States), there has been only one published case report of liver injury with symptoms and jaundice attributed to its use (Case 1). Thus, acute symptomatic liver injury due to tolterodine must be very rare, if it occurs at all.
Likelihood score: D (possible, very rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which tolterodine might cause liver injury unknown. It is metabolized in the liver by microsomal P450 enzymes, predominantly CYP 3A4 and 2D6. Despite this, drug-drug interactions are uncommon. A major reason for its safety may relate to the low daily dose.
Drug Class: Anticholinergic Agents
CASE REPORT
Case 1. Mixed hepatitis arising after 18 days of tolterodine therapy.(1)
An 81 year old woman with overactive bladder syndrome developed fever, fatigue, and nausea 18 days after starting tolterodine (2 mg twice daily). Her symptoms were initially attributed to a cold, but two days later when she had not improved, she discontinued tolterodine. The following day, she sought medical care and was found to have fever and abnormal liver tests including an ALT of 479 U/L, AST 301 U/L, Alk P 389 U/L, and GGT 292 U/L. She had no history of liver disease, exposures to viral hepatitis or known drug allergies. Her other medications included flunitrazepam once daily and diclofenac a few times weekly for arthritis, both of which she had taken for several years. Physical examination revealed mild fever (38.4oC) without rash, lymphadenopathy or abdominal tenderness. A liver ultrasound was normal without evidence of biliary obstruction. Over the next few days, she developed mild jaundice (bilirubin 2.4 mg/dL) and a leukocytosis and eosinophilia (8%). Because of concern about sepsis, she was treated with ciprofloxacin for 4 days, but blood cultures were negative, and liver tests had begun to improve. Two weeks after stopping tolterodine, she was asymptomatic and liver tests were only mildly elevated. When seen in follow up 4 weeks after stopping tolterodine, all liver tests were normal.
Key Points
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Tolterodine started (2 mg twice daily) | |||||
| 10 days | pre | Normal | Normal | Asymptomatic | |
| 18 days | 20 | Fatigue, nausea, and fever | |||
| 20 days | 0 | Tolterodine stopped | |||
| 21 days | 1 day | 479 | 389 | Normal | |
| 25 days | 5 days | 2.4 | |||
| 34 days | 2 weeks | 82 | 244 | Normal | Asymptomatic |
| 48 days | 4 weeks | Normal | Normal | Normal | |
| Normal Values* | <40 | <125 | <1.2 | ||
- *
Imputed upper limit of normal values.
Comment
This patient was found to have symptoms of fever, fatigue, nausea and abdominal upset with a “mixed” pattern of serum enzyme elevations 2 to 3 weeks after starting tolterodine. The aminotransferase levels were approximately 12 times and alkaline phosphatase 3 times the upper limit of normal, and both had been normal when tested several weeks earlier (and while on tolterodine). While the presentation and course of illness were entirely compatible with a hypersensitivity reaction accompanied by mild hepatic injury, other possibilities were not completely excluded. Helpful would have been mention of whether the ultrasound showed gallstones since temporary biliary obstruction from passing a gallstone can mimic a mild and transient episode of drug induced liver injury. In addition, follow up information on whether diclofenac was restarted without return of liver injury would be reassuring. Diclofenac is a common cause of drug induced liver injury while tolterodine is not, this being the only case report of liver injury due to tolterodine in the literature despite its availability and common use for more than 25 years (more than 1 million prescriptions were filled in the United States in 2020).
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tolterodine – Generic, Detrol®
DRUG CLASS
Anticholinergic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Tolterodine | 124937-51-5 | C22-H31-N-O |
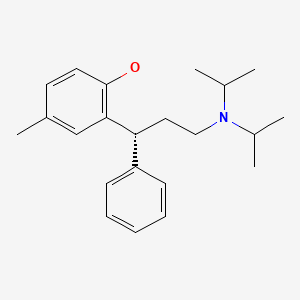
|
CITED REFERENCE
- 1.
- Schlienger RG, Keller MJ, Krähenbühl S. Tolterodine-associated acute mixed liver injury. Ann Pharmacother 2002; 36 (5): 817-9. [PubMed: 11978158]
ANNOTATED BIBLIOGRAPHY
References updated: 12 July 2023
Abbreviations: ER, extended release.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999 before the availability of darifenacin and other therapies of overactive bladder syndrome).
- Brown JH, Brandl K, Wess J. Therapeutic uses of muscarinic receptor antagonists: Muscarinic receptor agonists and antagonists. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 156-9.(Textbook of pharmacology and therapeutics).
- Schlienger RG, Keller MJ, Krähenbühl S. Tolterodine-associated acute mixed liver injury. Ann Pharmacother 2002; 36 (5): 817-9. [PubMed: 11978158](81 year old woman developed fatigue and nausea 18 days after starting tolterodine for overactive bladder syndrome [bilirubin 2.4 mg/dL, ALT 479 U/L, Alk P 389 U/L, eosinophils 8%], resolving within 4 weeks of stopping: Case 1).
- Chapple CR, Rechberger T, Al-Shukri S, Meffan P, Everaert K, Huang M, Ridder A; YM-905 Study Group. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int. 2004;93:303-10. [PubMed: 14764127](Among 1077 adults with overactive bladder syndrome treated with solifenacin [5 and 10 mg], tolterodine [4 mg], or placebo daily for 12 weeks, both agents led to a significant decrease in numbers of micturitions and episodes of urgency and incontinence, with similar rates of adverse events, and “there were no clinically relevant changes in … laboratory values”).
- Chapple CR, Martinez-Garcia R, Selvaggi L, Toozs-Hobson P, Warnack W, Drogendijk T, Wright DM, et al.; STAR study group. A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial. Eur Urol. 2005;48:464-70. [PubMed: 15990220](Among 1200 adults with overactive bladder syndrome treated with solifenacin [5 or 10 mg] or tolterodine ER [4 mg] once daily for 12 weeks, numbers of daily micturitions decreased in both groups [-2.5 vs -2.2], and adverse event rates were similar [dry mouth 18% vs 15%, constipation 3.2% vs 1.3%, blurred vision 0.7% vs 1.7%], but no mention of ALT elevations or hepatotoxicity).
- Novara G, Galfano A, Secco S, D'Elia C, Cavalleri S, Ficarra V, Artibani W. A systematic review and meta-analysis of randomized controlled trials with antimuscarinic drugs for overactive bladder. Eur Urol 2008; 54: 740-63. [PubMed: 18632201](Systematic review of efficacy and safety of drugs for overactive bladder including tolterodine, propiverine, solifenacin, darifenacin, fesoterodine and oxybutynin; common side effects included dry mouth and constipation; hepatotoxicity and ALT elevations were not mentioned).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to anticholinergics or drugs for overactive bladder).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to anticholinergics or drugs for overactive bladder syndrome).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](Among 624,673 adverse event reports in children between 2000 and 2006 in the WHO VigiBase, 1% were hepatic, but no anticholinergic was listed among the 41 most frequently implicated agents).
- Shamliyan T, Wyman JF, Ramakrishnan R, Sainfort F, Kane RL. Benefits and harms of pharmacologic treatment for urinary incontinence in women: a systematic review. Ann Intern Med 2012; 156: 861-74. [PubMed: 22711079](Systematic review of the safety and efficacy of drugs used for urinary incontinence including fesoterodine, tolterodine, oxybutynin, solifenacin and trospium; most had modest effectiveness; hepatotoxicity was not mentioned).
- Khullar V, Amarenco G, Angulo JC, Cambronero J, Høye K, Milsom I, Radziszewski P, et al. Efficacy and tolerability of mirabegron, a β(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European-Australian phase 3 trial. Eur Urol 2013; 63: 283-95. [PubMed: 23182126](Among 1978 patients with overactive bladder syndrome treated with once daily mirabegron [50 or 100 mg], tolterodine [4 mg] or placebo for 12 weeks, adverse event rates were similar among groups, and "changes in...serum chemistry parameters...were small and consistent across treatment groups").
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to mirabegron or drugs for overactive bladder syndrome).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, but none were attributed to drugs used for overactive bladder syndrome).
- Maman K, Aballea S, Nazir J, Desroziers K, Neine ME, Siddiqui E, Odeyemi I, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014;65:755-65. [PubMed: 24275310](Systematic review of literature on medical therapies for overactive bladder identified 44 controlled trials demonstrating similar efficacy among 6 anticholinergics and a single beta-3 adrenergic agonist [mirabegron] when compared to placebo, but less dry mouth with mirabegron than with anticholinergic agents; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN Prospective Study. Gastroenterology 2015; 148: 1340-1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to mirabegron or other agents for overactive bladder syndrome).
- Thiagamoorthy G, Cardozo L, Srikrishna S. Drug therapy for an overactive bladder. Womens Health (Lond) 2015; 11: 445-8. [PubMed: 26238677](Overactive bladder is defined as urinary urgency, usually with frequency and nocturia with or without incontinence in the absence of infection or other known cause, medical therapy being use of anticholinergics or beta-3 adrenergic receptor agonists such as mirabegron or vibegron which have fewer side effects than typical anticholinergics).
- Staskin D, Frankel J, Varano S, Shortino D, Jankowich R, Mudd PN Jr. International phase III, randomized, double-blind, placebo and active controlled study to evaluate the safety and efficacy of vibegron in patients with symptoms of overactive bladder: EMPOWUR. J Urol. 2020;204:316-324. [PubMed: 32068484](Among 1518 adults with overactive bladder treated with vibegron [75 mg], tolterodine [4 mg], or placebo once daily for 12 weeks, daily micturitions decreased by 1.8 episodes with the two medications vs 1.4 with placebo, while adverse events were uncommon [39% and 39% vs 33%] and ALT elevations were rare [0.2% and 0.2% vs 0.4%] and there were no hepatic severe adverse events or discontinuations).
- Drugs for overactive bladder. Med Lett Drugs Ther. 2023;65:41-45. [PubMed: 36897601](Concise review of drugs approved for therapy of overactive bladder in the US including anticholinergic agents [darifenacin, fesoterodine, oxbutynin, solifenacin, tolterodine and trospium] and beta-3 adrenergic receptor agonists [mirabegron and vibegron], including clinical efficacy, safety, and costs; no mention of ALT elevations or hepatotoxicity of any of the agent discussed).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Which anticholinergic drug for overactive bladder symptoms in adults.[Cochrane Database Syst Rev. 2012]Review Which anticholinergic drug for overactive bladder symptoms in adults.Madhuvrata P, Cody JD, Ellis G, Herbison GP, Hay-Smith EJ. Cochrane Database Syst Rev. 2012 Jan 18; 1:CD005429. Epub 2012 Jan 18.
- Review Which anticholinergic drug for overactive bladder symptoms in adults.[Cochrane Database Syst Rev. 2005]Review Which anticholinergic drug for overactive bladder symptoms in adults.Hay-Smith J, Herbison P, Ellis G, Morris A. Cochrane Database Syst Rev. 2005 Jul 20; (3):CD005429. Epub 2005 Jul 20.
- Effectiveness and tolerability of extended-release oxybutynin vs extended-release tolterodine in women with or without prior anticholinergic treatment for overactive bladder.[Int Urogynecol J Pelvic Floor ...]Effectiveness and tolerability of extended-release oxybutynin vs extended-release tolterodine in women with or without prior anticholinergic treatment for overactive bladder.Anderson RU, MacDiarmid S, Kell S, Barada JH, Serels S, Goldberg RP. Int Urogynecol J Pelvic Floor Dysfunct. 2006 Sep; 17(5):502-11. Epub 2006 May 3.
- Treatments for overactive bladder: focus on pharmacotherapy.[J Obstet Gynaecol Can. 2012]Treatments for overactive bladder: focus on pharmacotherapy.Geoffrion R, UROGYNAECOLOGY COMMITTEE. J Obstet Gynaecol Can. 2012 Nov; 34(11):1092-1101.
- Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial.[JAMA. 2006]Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial.Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. JAMA. 2006 Nov 15; 296(19):2319-28.
- Tolterodine - LiverToxTolterodine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...