NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Topiramate is a unique antiseizure medication that is widely used in treatment of partial and generalized seizures as well as for bipolar disorder, migraines and weight loss. Topiramate has been rarely associated with hepatic injury and largely when used in combination with other anticonvulsant medications.
Background
Topiramate (toe pyre' a mate) is a sulfamate-substituted monosaccharide and belongs to an anticonvulsant class of its own. Topiramate is believed to act by reducing sodium channel currents and enhancing gamma aminobutyric acid A (GABA-A) receptor activity. Topiramate was approved for use in epilepsy in the United States in 1996 and it is still widely used with more than 10 million prescriptions filled yearly. Its current indications are for prevention and management of partial onset and generalized seizures used either as monotherapy or in combination with other anticonvulsants. Topiramate is also used for migraine, for bipolar disorder and in fixed combination with phentermine (Qsymia) for weight loss. The recommended starting dose in adults is 25 mg twice daily, escalating at weekly intervals to a maximum of 200 mg twice daily. Topiramate is available in 25, 50, 100 and 200 mg tablets in multiple generic forms and under the brand name Topromax. Pediatric formulations as sprinkle capsules are available in doses of 15 and 25 mg. Common side effects include dizziness, somnolence, paresthesias, change in taste, anorexia, weight loss, itching, difficulty concentrating and nervousness. Uncommon but potentially severe adverse reactions include acute myopia, acute glaucoma, visual field defects, metabolic acidosis, suicidal ideation and behaviors, hyperammonemia, kidney stones, hyperthermia and hypothermia.
Hepatotoxicity
Prospective studies suggest that less than 1% of subjects develop elevations in serum aminotransferase levels during long term topiramate therapy. Clinically apparent hepatotoxicity from topiramate is quite rare and usually arises in patients receiving multiple other anticonvulsants. Topiramate is metabolized by CYP 3A4 and may increase the risk of valproate or other anticonvulsant hepatotoxicity. A distinctive syndrome is the development of lethargy, weakness with marked serum aminotransferase elevations and hyperammonemia arising within 2 to 3 weeks of the addition (or dose increase) of topiramate to long term valproate therapy. While valproate alone can cause a similar syndrome, it appears much more common (~1%) with the combination than with valproate alone (~0.1%). This syndrome has several features suggestive of Reye syndrome (hyperammonemia, hypoglycemia, rapid reversal of injury) and in many instances is preceded by a acute viral illness. Topiramate by itself has only rarely been linked to clinically apparent liver injury and the clinical features and course of injury have not been well defined. Topiramate has not been linked to cases of the anticonvulsant hypersensitivity syndrome and is considered a safe alternative in patients with that syndrome.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of topiramate hepatotoxicity is thought to be due to its effects in inducing CYP 3A4 or inhibiting CYP 2C19 and possibly through the effects of a toxic metabolic intermediate. Cases with lactic acidosis and hyperammonemia may be caused by mitochondrial injury or dysfunction.
Outcome and Management
Topiramate hepatotoxicity is usually attributed to its effects on the metabolism of other anticonvulsants, and as such is rapidly reversible within a few days of either drug being stopped. Vanishing bile duct injury and chronic injury from topiramate therapy has not been reported.
Drug Class: Anticonvulsants
See also: Phentermine-Topiramate
CASE REPORT
Case 1. Acute liver failure with lactic acidosis attributed to topiramate.(1)
An 11 year old boy with cerebral palsy, severe neurologic deficits and seizures on long term topiramate (10 years) developed diarrhea and somnolence and was found to have acute liver failure. His other medications included phenobarbital, diazepam and baclofen, all of which he had taken for years. There was no mention of recent illness, fever, influenza-like symptoms or aspirin use. On presentation the child was comatose and unresponsive. Serum bilirubin was 5.9 mg/dL, ALT 7890 U/L, AST 5666 U/L, GGT 243 U/L, ammonia 1350 ug/dL, and INR 10.8. In addition, he had hypovolemic shock, renal dysfunction (creatinine 2.2 mg/dL) and lactic acidosis with an arterial pH 7.1 and serum lactate of 18 mmol/L. Topiramate was stopped and he was resuscitated with fluids and fresh frozen plasma. Tests for hepatitis A, B and C and for other viral infections such as Epstein Barr virus, cytomegalovirus and adenoviruses were negative. Autoantibodies were not detectable and imaging of the liver showed no abnormalities. He improved rapidly and a liver biopsy showed marked microvesicular steatosis [50% of hepatic parenchyma] and acute hepatitis. While recovery from the liver injury was complete, he was left with further neurologic compromise and was ventilator dependent.
Key Points
| Medication: | Topiramate (dose not given) |
|---|---|
| Pattern: | Hepatocellular (R~100) |
| Severity: | 4+ (jaundice and prolonged INR) |
| Latency: | 10 years |
| Recovery: | Several weeks |
| Other medications: | Phenobarbital, diazepam, baclofen |
Comment
A child with seizure disorder on long term topiramate developed an acute Reye’s syndrome-like illness with hyperammonemia, lactic acidosis, microvesicular fat and severe hepatic dysfunction (LASH). While the authors attributed the liver injury to topiramate, the long latency and precipitous nature of the illness suggests that it was unrelated and possibly due to an acute exposure to aspirin during a preceding, intercurrent viral illness. Another possibility is that topiramate can act like aspirin in triggering Reye’s syndrome, similar to what has been described with valproate and amiodarone. Indeed, several instances of acute liver failure possibly compatible with Reye’s syndrome have been described in young patients taking topiramate.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Topiramate – Generic, Topamax®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
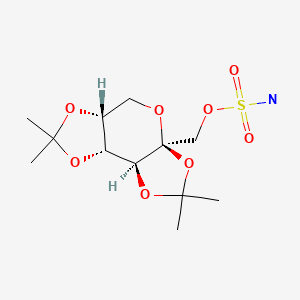
| Topiramate | 97240-79-4 | C12-H21-N-O8-S |
|
CITED REFERENCE
- 1.
- Tsien MZ, Cordova J, Qadir A, Zhao L, Hart J, Azzam R. Topiramate-Induced acute liver failure in a pediatric patient: a case report and review of literature. J Pediatr Gastroenterol Nutr. 2016;63:e37–8. [PubMed: 25207478]
ANNOTATED BIBLIOGRAPHY
References updated: 30 July 2020
Abbreviations used: CNS, central nervous system; DRESS, drug rash with eosinophilia and systemic symptoms; SJS/TEN, Stevens-Johnson syndrome and toxic epidermal necrolysis.
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999; topiramate is not discussed).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-42.(Review of anticonvulsant induced liver injury; topiramate is not discussed).
- Smith MD, Metcalf CS, Wilcox KS. Pharmacology of the epilepsies. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 303-26.(Textbook of pharmacology and therapeutics).
- Wallace SJ. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf. 1996;15:378–93. [PubMed: 8968693](Systematic review; ALT elevations occur in 4% of children on phenytoin, 6% on valproate, 1% on carbamazepine, but none reported on tiagabine or gabapentin; no mention of hepatotoxicity of topiramate).
- Langtry HD, Gillis JC, Davis R. Topiramate. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in the management of epilepsy. Drugs. 1997;54:752–73. [PubMed: 9360061](Summary of prelicensure studies of pharmacokinetics, efficacy and safety of topiramate; shown to be effective in doses of 400-1000 mg alone and in lower doses as adjunctive therapy for epilepsy; major side effects are central nervous system related, including dizziness, somnolence, nervousness, paresthesia, ataxia and confusion; it also causes renal stones, anorexia and weight loss; no mention of ALT abnormalities or hepatotoxicity).
- Bjøro K, Gjerstad L, Bentdal O, Osnes S, Schrumpf E. Topiramate and fulminant liver failure. Lancet. 1998;352:1119. [PubMed: 9798593](39 year old woman on chronic carbamazepine therapy developed progressive stupor 4 months after starting topiramate and shortly after a dose increase [300 mg/day], with hypoglycemia and acidosis [bilirubin not given, ALT 1350 rising to 10,000 U/L], coagulopathy and coma, requiring liver transplantation, explant showed massive centrilobular necrosis).
- Sachdeo RC. Topiramate. Clinical profile in epilepsy. Clin Pharmacokinet. 1998;34:335–46. [PubMed: 9592618](Review of pharmacology, clinically efficacy and safety of topiramate; common side effects are dizziness, slowed thinking, somnolence, ataxia, fatigue, confusion and paresthesias; long term therapy may cause weight change and nephrolithiasis; no mention of ALT elevations or hepatotoxicity).
- Hamer HM, Morris HH. Hypersensitivity syndrome to antiepileptic drugs: a review including new anticonvulsants. Cleve Clin J Med. 1999;66:239–45. [PubMed: 10199060](Clinical review of anticonvulsant hypersensitivity syndrome which occurs in 1-5 per 10,000 users of the major aromatic anticonvulsants, higher risk in African Americans and affected siblings; liver involvement common, but most cases anicteric; other manifestations include facial edema, lymphadenopathy, bone marrow aplasia, pseudolymphoma, thyroiditis, interstitial nephritis).
- Faught E. Clinical studies of topiramate. Drugs Today (Barc). 1999;35:49–57. [PubMed: 12973409](Review of results of clinical trials of topiramate; most common side effects were CNS symptoms such as dizziness and somnolence; "There have been no cases of severe liver dysfunction or damage from topiramate").
- Doan RJ, Clendenning M. Topiramate and hepatotoxicity. Can J Psychiatry. 2000;45:937–8. [PubMed: 11190367](21 year old woman with bipolar disorder was found to have liver test abnormalities two months after adding topiramate to chronic therapy with valproate, benztropine, and risperidone [bilirubin not mentioned; ALT 627 rising to 1909 U/L, Alk P 81 U/L, INR normal, valproate levels normal], resolving within 1 month and no recurrence on restarting valproate without topiramate).
- Longin E, Teich M, Koelfen W, König S. Topiramate enhances the risk of valproate-associated side effects in three children. Epilepsia. 2002;43:451–4. [PubMed: 11952778](3 children, ages 1-9 years on valproate for few months to 5 years developed apathy and hypothermia 4-8 weeks after adding topiramate with ammonia elevations [one with ALT elevation to 400 U/L as well], rapidly reversed by stopping either agent, positive rechallenge in one. Topiramate appears to enhance risk of hyperammonemia due to valproate).
- Langman LJ, Kaliciak HA, Boone SA. Fatal acute topiramate toxicity. J Anal Toxicol. 2003;27:323–4. [PubMed: 12908948](44 year old woman was found dead with high levels of topiramate in blood and liver; death ascribed to acute topiramate overdose; no mention of liver injury).
- Bumb A, Diederich N, Beyenburg S. Adding topiramate to valproate therapy may cause reversible hepatic failure. Epileptic Disord. 2003;5:157–9. [PubMed: 14684351](51 year old woman with resistant epilepsy developed confusion and liver injury after addition of topiramate to chronic valproate therapy [bilirubin not given, ALT 464 U/L, GGT 569 U/L, ammonia 88 μg/dL], resolving upon stopping valproate: topiramate likely changed pharmacokinetics of valproate).
- Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDavid C, Mason A, et al. Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technol Assess. 2005;9:1–157. iii-iv. [PubMed: 15842952](Systematic review of anticonvulsant medications including assessment of serious, rare and long term adverse events, serious adverse events from topiramate were limited to neurological effects; no mention of hepatotoxicity).
- Topal F, Ozaslan E, Akbulut S, Küçükazman M, Yüksel O, Altiparmak E. Methylprednisolone-induced toxic hepatitis. Ann Pharmacother. 2006;40:1868–71. [PubMed: 16926305](Acute hepatic injury arising after 7 days of self-prescribed methylprednisolone [32 mg/day] in a patient on long term topiramate [bilirubin 10 mg/dL, ALT 2478 U/L, Alk P 138 U/L], with rapid recovery with stopping both; no recurrence on restarting topiramate).
- LaRoche SM. A new look at the second-generation antiepileptic drugs: a decade of experience. Neurologist. 2007;13:133–9. [PubMed: 17495757](Review of second generation anticonvulsants approved since 1994 including felbamate, gabapentin, lamotrigine, topiramate, tiagabine, levetiracetam, oxcarbazepine, zonisamide and pregabalin; no mention of liver toxicity from topiramate).
- Nicolai J, Gunning B, Leroy PL, Ceulemans B, Vles JS. Acute hepatic injury in four children with Dravet syndrome: valproic acid, topiramate or acetaminophen? Seizure. 2008;17:92–7. [PubMed: 17697789](4 children with severe infantile myoclonic epilepsy [Dravet syndrome: SCN1A+] on chronic therapy with topiramate and valproate had sudden and transient onset of marked elevations in ALT [2209, 5898, 280 and 480 U/L] and hyperammonemia or coagulopathy without jaundice shortly after febrile illness and/or use of acetaminophen; possibly Reye syndrome due to valproate and topiramate, rapid recovery despite continuing anticonvulsants).
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, et al. Topiramate for Alcoholism Advisory Board. Topiramate for Alcoholism Study Group. Improvement of physical health and quality of life of alcohol-dependent individuals with topiramate treatment: US multisite randomized controlled trial. Arch Intern Med. 2008;168:1188–99. [PubMed: 18541827](Randomized controlled trial of topiramate vs placebo in 371 alcohol dependent patients found ALT levels decreased more in treated than placebo group; one patient in each group had de novo ALT elevations).
- Chung AM, Eiland LS. Use of second-generation antiepileptic drugs in the pediatric population. Paediatr Drugs. 2008;10:217–54. [PubMed: 18590343](Extensive review of antiepileptic drugs and their use in children; no mention of topiramate).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, valproate accounted for 6 cases, lamotrigine 5, phenytoin 5, gabapentin and topiramate 1 each; however, the case initially linked to topiramate was later found to be due to acute hepatitis C).
- Del Val Antoñana A, Ortiz Polo I, Andrade Bellido RJ. Gastroenterol Hepatol. 2010;33:148–9. [Topiramate-induced acute hepatitis] Spanish. [PubMed: 19800146](16 year old girl developed fatigue and abdominal pain 10 weeks after starting topiramate [bilirubin 1.3 mg/dL, ALT 8030 U/L, Alk P 135 U/L, INR 2.6, glucose 52 mg/dL], resolving rapidly, within 2 weeks of stopping).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 11 linked to anticonvulsants, but none to topiramate).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; 35 were attributed to anticonvulsants, but none to topiramate].
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N., Drug-Induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN Prospective Study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with drug induced liver injury enrolled in a prospective US database between 2004 and 2008, 8 were due to anticonvulsants [lamotrigine in 3, valproate in 3, phenytoin in 1 and carbamazepine in 1], but none to topiramate).
- Noh Y, Kim DW, Chu K, Lee ST, Jung KH, Moon HJ, Lee SK. Topiramate increases the risk of valproic acid-induced encephalopathy. Epilepsia. 2013;54:e1–4. [PubMed: 22691153](Retrospective analysis of 8,372 Korean patients with epilepsy treated with valproate at a single referral hospital between 2001 and 2009 found 1236 who also received topiramate; hyperammonemic coma developed in 0.6% of those on the combination compared to only 0.06% on valproate alone).
- Gaitatzis A, Sander JW. The long-term safety of antiepileptic drugs. CNS Drugs. 2013;27:435–55. [PubMed: 23673774](Review of long term safety of commonly used anticonvulsants; topiramate hepatotoxicity is not mentioned).
- Drugs for epilepsy. Treat Guidel Med Lett. 2013;11:9–18. Erratum in Treat Guidel Med Lett 2013; 11: 112. [PubMed: 23348233](Concise review of indications and side effects of anticonvulsants; topiramate is used for partial and primary generalized tonic-clonic seizures, both as monotherapy and adjunctive therapy in adults and children; adverse events include drowsiness, dizziness, headache and ataxia; mentions that liver failure has been reported).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 1 attributed to phenytoin among only 410 persons receiving the drug in Iceland; no case was attributed to topiramate).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 7 [4%] of which were attributed to anticonvulsants, 3 to phenytoin, 3 to valproate and 1 to carbamazepine, but none to topiramate).
- Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145–61. [PubMed: 24879980](Review of drug induced liver injury caused by several drug classes including antiepileptics, which account for 2-11% of all cases in various registries; a common presentation is with DRESS particularly with the older agents such as carbamazepine, phenytoin and phenobarbital and often associated with reactivation of human herpes virus 6 or 7; no mention of topiramate hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were due to anticonvulsants including 12 due to phenytoin, 9 lamotrigine, 7 valproate, 4 carbamazepine, 3 gabapentin, 2 topiramate and 1 each for ethosuximide, fosphenytoin, and pregabalin).
- Tsien MZ, Cordova J, Qadir A, Zhao L, Hart J, Azzam R. Topiramate-Induced acute liver failure in a pediatric patient: a case report and review of literature. J Pediatr Gastroenterol Nutr. 2016;63:e37–8. [PubMed: 25207478](11 year old boy with cerebral palsy and epilepsy on topiramate for 10 years developed somnolence and was found to have acute liver failure, lactic acidosis and shock [bilirubin 5.9 mg/dL, ALT 7890 U/L, GGT 293 U/L, INR 10.8, ammonia 1350 µg/dL], liver biopsy showing microvesicular steatosis, recovering with supportive care and stopping topiramate; Case 1).
- Chalasani N, Reddy KRK, Fontana RJ, Barnhart H, Gu J, Hayashi PH, Ahmad J, Stolz A, Navarro V, Hoofnagle JH. Idiosyncratic drug induced liver injury in African-Americans is associated with greater morbidity and mortality compared to Caucasians. Am J Gastroenterol. 2017;112:1382–8. [PMC free article: PMC5667647] [PubMed: 28762375](Among subjects enrolled in a US prospective database of drug induced liver injury, causes more frequent in African Americans than Caucasians were trimethoprim/sulfamethoxazole, methyldopa, phenytoin and allopurinol, and African Americans were more likely to have severe skin adverse events [2.1% vs 0.4%], fatal or transplantation outcomes [10% vs 6%] as well as chronic injury [24% vs 16%]).
- Vidaurre J, Gedela S, Yarosz S. Antiepileptic drugs and liver disease. Pediatr Neurol. 2017;77:23–36. [PubMed: 29097018](Review of the use of anticonvulsants in patients with liver disease recommends use of agents that have little hepatic metabolism such as levetiracetam, lacosamide, topiramate, gabapentin and pregabalin, levetiracetam being an "ideal" first line therapy for patients with liver disease because of its safety and lack of pharmacokinetic interactions).
- Drugs for epilepsy. Med Lett Drugs Ther. 2017;59(1526):121–30. [PubMed: 28746301](Concise review of the drugs available for therapy of epilepsy lists topiramate as approved as monotherapy or adjunctive therapy for partial and primary generalized tonic-clonic seizures, common adverse events being drowsiness, dizziness, headache and ataxia and mentions that hepatic failure has been reported).
- Khivsara A, Raj JP, Hegde D, Rao M. Topiramate-induced acute liver injury: A rare adverse effect. Indian J Pharmacol. 2017;49:254–6. [PMC free article: PMC5637137] [PubMed: 29033486](31 year old man with alcoholism and withdrawal seizures was restarted on topiramate, and aminotransferase levels worsened within 3 days and improved after discontinuation with no change in symptoms or bilirubin levels [ALT initially 161 rising to 675 and falling thereafter to 277 U/L, Alk P initially 101 rising to 121 U/L]).
- Borrelli EP, Lee EY, Descoteaux AM, Kogut SJ, Caffrey AR. Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: An analysis of the US Food and Drug Administration Adverse Event Reporting System. Epilepsia. 2018;59:2318–24. [PMC free article: PMC6420776] [PubMed: 30395352](Review of adverse event reports to the FDA between 2014 and 2018 identified ~2.9 million reports, 1034 for Stevens-Johnson syndrome or toxic epidermal necrolysis, the most common class of drugs being anticonvulsants with 17 of 34 having at least one report, those most frequently linked being lamotrigine [n=106], carbamazepine [22], levetiracetam [14], phenytoin [14], valproate [9], clonazepam [8], zonisamide [7], gabapentin [4], pregabalin [4], oxcarbazepine [3]; no mention of topiramate).
- Cano-Paniagua A, Amariles P, Angulo N, Restrepo-Garay M. Epidemiology of drug-induced liver injury in a University Hospital from Colombia: Updated RUCAM being used for prospective causality assessment. Ann Hepatol. 2019;18:501–7. [PubMed: 31053545](Among 286 patients with liver test abnormalities seen in a single hospital in Colombia over a 1 year period, 17 were diagnosed with drug induced liver injury, the most common cause being antituberculosis therapy [n=6] followed by anticonvulsants [n=3, 1 each due to phenytoin, gabapentin and valproate]; none were attributed to topiramate).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Effects on weight and outcome of long-term olanzapine-topiramate combination treatment in bipolar disorder.[J Clin Psychopharmacol. 2004]Effects on weight and outcome of long-term olanzapine-topiramate combination treatment in bipolar disorder.Vieta E, Sánchez-Moreno J, Goikolea JM, Colom F, Martínez-Arán A, Benabarre A, Corbella B, Torrent C, Comes M, Reinares M, et al. J Clin Psychopharmacol. 2004 Aug; 24(4):374-8.
- Review The evolving role of topiramate among other mood stabilizers in the management of bipolar disorder.[Bipolar Disord. 2001]Review The evolving role of topiramate among other mood stabilizers in the management of bipolar disorder.Chengappa KN, Gershon S, Levine J. Bipolar Disord. 2001 Oct; 3(5):215-32.
- Review Topiramate for acute affective episodes in bipolar disorder.[Cochrane Database Syst Rev. 2006]Review Topiramate for acute affective episodes in bipolar disorder.Vasudev K, Macritchie K, Geddes J, Watson S, Young A. Cochrane Database Syst Rev. 2006 Jan 25; (1):CD003384. Epub 2006 Jan 25.
- Topiramate as add-on treatment for patients with bipolar mania.[Bipolar Disord. 1999]Topiramate as add-on treatment for patients with bipolar mania.Chengappa KN, Rathore D, Levine J, Atzert R, Solai L, Parepally H, Levin H, Moffa N, Delaney J, Brar JS. Bipolar Disord. 1999 Sep; 1(1):42-53.
- The anticonvulsant effect of chronic treatment with topiramate after pilocarpine-induced status epilepticus is accompanied by a suppression of comorbid behavioral impairments and robust neuroprotection in limbic regions in rats.[Epilepsy Behav. 2022]The anticonvulsant effect of chronic treatment with topiramate after pilocarpine-induced status epilepticus is accompanied by a suppression of comorbid behavioral impairments and robust neuroprotection in limbic regions in rats.Shishmanova-Doseva M, Atanasova D, Ioanidu L, Uzunova Y, Atanasova M, Peychev L, Tchekalarova J. Epilepsy Behav. 2022 Sep; 134:108802. Epub 2022 Jul 2.
- Topiramate - LiverToxTopiramate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...