NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tinidazole is an orally available, broad spectrum antimicrobial agent used in the treatment of bacterial, protozoal and parasitic infections. Tinidazole is a nitroimidazole similar to metronidazole and is likely to have a similar spectrum and frequency of side effects, including a low rate of serum enzyme elevations during therapy and rare instances of clinically apparent acute liver injury.
Background
Tinidazole (tye nid' a zole) is an oral, broad spectrum antimicrobial that has activity against bacteria as well as several parasites such as Entamoeba histolytica, Giardia duodenalis and Trichomonas vaginalis. Tinidazole is a nitroimidazole similar to metronidazole and is activated intracellularly by bacterial or parasitic enzymes to a radical anion, which damages large protein molecules and DNA. Tinidazole was approved for use in the United States in 2004 and current indications are for treatment of trichomoniasis, giardiasis, amebiasis and bacterial vaginosis. Tinidazole is available in tablets of 250 and 500 mg and the dose varies by indication, being 2 grams as a single oral dose in adults with trichomoniasis and giardiasis, and for 3 to 5 days for moderate or severe forms of amebiasis. Oral tinidazole is generally well tolerated if being used generally for only 1 to 5 days. In most comparison studies, its efficacy and spectrum of activity were found to be similar to those of metronidazole, but it was often better tolerated. Side effects can include metallic or bitter taste, fatigue, dizziness, headache, dyspepsia, nausea, and a disulfiram-like response to alcohol. Rare, but potential serious adverse events include allergic reactions, anaphylaxis and toxic megacolon.
Hepatotoxicity
Tinidazole is typically given for a few days only, but serum enzyme elevations have been reported with its use, and serum enzyme elevations during therapy is listed as a possible adverse event in the product label. Tinidazole is also capable of causing anaphylactic and allergic reactions including urticaria, angioedema and bronchospasm, reactions which can be associated with minor serum enzyme elevations. Tinidazole, despite considerable use worldwide, has not been linked convincingly to instances of clinically apparent liver injury with jaundice.
Likelihood score: E* (suspected but unproven rare cause of clinically apparent liver injury)
Mechanism of Injury
The cause of rare instances of serum enzyme elevations with tinidazole therapy is unknown, but is likely to be immunologically mediated. Tinidazole is extensively metabolized in the liver largely via CYP 3A4 and can lead to significant drug-drug interactions.
Outcome and Management
The severity of the liver injury linked to tinidazole therapy has been invariably mild and self-limited, usually with transient, asymptomatic serum enzyme elevations accompanying hypersensitivity reactions. However, the use of tinidazole has been limited and it is typically given for 1 to 5 days only. In instances of allergic reactions or anaphylaxis, rechallenge should be avoided. There is no information to suggest that there is cross sensitivity to hypersensitivity reactions or hepatic injury between tinidazole and other antimicrobial agents; indeed, some patients have had severe hypersensitivity reactions to tinidazole after tolerating a course of metronidazole.
Drug Class: Antiinfective Agents
Other Drugs in the Subclass, Nitroimidazoles: Benznidazole, Metronidazole, Secnidazole
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tinidazole – Generic, Tindamax®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
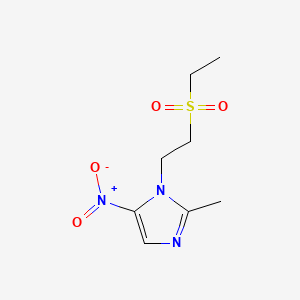
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Tinidazole | 19387-91-8 | C8-H13-N3-O4-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 February 2020
- Zimmerman HJ. Amebicides. Hepatic injury from antimicrobial agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, p. 592.(Expert review of liver injury published in 1999 mentions that metronidazole has only rarely been incriminated in causing liver injury).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-82.(Review of hepatotoxicity of antibiotics; does not discuss tinidazole).
- Wetzel DM, Phillips MA. Chemotherapy of protozoal infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 987-1000.(Textbook of pharmacology and therapeutics).
- Rosenthal PJ. Antiprotozoal drugs. In, Katzung BG, Masters SB, Trevor AJ. Basic and clinical pharmacology. 12th ed. New York: McGraw Hill, 2012, pp. 915-36.(Textbook of pharmacology and therapeutics, mentions that tinidazole has similar activity as metronidazole, but better toxicity profile and simpler dosing regimens).
- McEwen J. Hypersensitivity reactions to tinidazole (Fasigyn). Med J Aust. 1983;1(11):498–9. [PubMed: 6843436](Report of 10 instances of hypersensitivity reactions to tinidazole from an Australian registry, all arising within 24 hours of the first dose, 9 being severe and including generalized urticaria, facial or laryngeal edema, hypotension, bronchospasm and dyspnea; no mention of ALT elevations or jaundice).
- Howard K. Tinidazole and hepatitis. Med J Aust. 1985;142:513. [PubMed: 3990620](4 year old girl with giardiasis developed peripheral and facial edema within 3 days of a single oral dose of tinidazole [bilirubin 0.5 mg/dL, AST 231 U/L, CK 1498 U/L], resolving within a few days and liver tests being normal two weeks later).
- Roe FJ. Safety of nitroimidazoles. Scand J Infect Dis Suppl. 1985;46:72–81. [PubMed: 3865353](Review of the safety of tinidazole and other nitroimidazoles mentions side effects of nausea, headaches, metallic taste, furred tongue, itching and skin rash and at least one report of transient serum enzyme elevations).
- Tinidazole (Tindamax) - a new anti-protozoal drug. Med Lett Drugs Ther. 2004;46(1190):70–2. [PubMed: 15375353](Concise summary of activity, efficacy, side effects and costs of tinidazole shortly after its approval in the US, mentions that side effects were similar to metronidazole; no mention of ALT elevations or hepatotoxicity).
- Fung HB, Doan TL. Tinidazole: a nitroimidazole antiprotozoal agent. Clin Ther. 2005;27:1859–84. [PubMed: 16507373](Thorough review of structure, mechanism of action, pharmacology, efficacy and side effects of tinidazole in protozoal disease and bacterial vaginitis; adverse events were reported in 9-11% of recipients, most commonly metallic taste and nausea; no mention of ALT elevations or hepatotoxicity).
- Singbal SS, Rataboli PV. Anaphylaxis and hypersensitivity syndrome reactions in increasing severity following repeated exposure to tinidazole. J Postgrad Med. 2005;51:243–4. [PubMed: 16333209](30 year old man developed Stevens Johnson syndrome within a few days of starting tinidazole and norfloxacin, and had recurrence after starting single doses of both and another a third time when he took a half pill of tinidazole alone, having previously tolerated metronidazole; no mention of liver involvement or ALT levels).
- Livengood CH 3rd, Ferris DG, Wiesenfeld HC, Hillier SL, Soper DE, Nyirjesy P, Marrazzo J, et al. Effectiveness of two tinidazole regimens in treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2007;110(2 Pt 1):302–9. [PubMed: 17666604](Among 235 women with bacterial vaginosis treated with tinidazole [1 g daily for 5 days or 2 g daily for 2 days] or placebo, cure rates were higher with tinidazole [36% and 27%] versus placebo [5%]; side effects included dysgeusia [26% and 40% vs 5%] and nausea and dizziness; tinidazole had "no clinically significant effect" on liver function tests).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US between 2004 and 2008, more than 100 agents were implicated and the most commonly implicated class of agents was antibiotics, but no case was attributed to tinidazole or metronidazole).
- Escobedo AA, Alvarez G, González ME, Almirall P, Cañete R, Cimerman S, Ruiz A, et al. The treatment of giardiasis in children: single-dose tinidazole compared with 3 days of nitazoxanide. Ann Trop Med Parasitol. 2008;102:199–207. [PubMed: 18348774](Among 166 children with giardiasis who were treated with tinidazole [3 g single dose] or nitazoxanide [twice daily for 3 days], cure rates were 90% vs 78% and there were only mild, transient side effects; no mention of ALT elevations or hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to tinidazole or metronidazole).
- Drugs for parasitic infections. 2nd ed. New Rochelle, NY: Medical Letter, Inc., 2010.(Thorough description of drugs for parasitic infections in adults and children as well as a table of their major side effects; tinidazole, like metronidazole, is considered a first line agent for treatment of amebiasis, giardiasis and trichomonas infections; side effects are listed as occasional [metallic taste, GI symptoms, rash] and rare [weakness]).
- Pasupuleti V, Escobedo AA, Deshpande A, Thota P, Roman Y, Hernandez AV. Efficacy of 5-nitroimidazoles for the treatment of giardiasis: a systematic review of randomized controlled trials. PLoS Negl Trop Dis. 2014;8:e2733. [PMC free article: PMC3953020] [PubMed: 24625554](Systematic review of 30 randomized controlled trials in 3930 patients comparing nitroimidazoles to placebo in giardiasis; mentions that harmful outcomes were uncommon and side effects reported more commonly than with placebo included only metallic taste and headache; no mention of ALT levels or hepatotoxicity).
- Blumenthal KG, Youngster I, Rabideau DJ, Parker RA, Manning KS, Walensky RP, Nelson SB. Peripheral blood eosinophilia and hypersensitivity reactions among patients receiving outpatient parenteral antibiotics. J Allergy Clin Immunol 2015; 136: 1288-94. e1. [PMC free article: PMC4640981] [PubMed: 25981739](Among 824 patients receiving outpatient parenteral antibiotic therapy who had concurrent white blood cell and differential counts, eosinophilia [>500 cells/uL] was found in 25%, being most common with vancomycin [30%], the penicillins [28%], carbapenems [28%], cephalosporins [22%], fluoroquinolones [21%], and less common with metronidazole [15%] and there was no association of eosinophilia with liver test abnormalities).
- Drugs for Helicobacter pylori infection. Med Lett Drugs Ther. 2017;59(1525):113–7. [PubMed: 28699931](Concise review of the efficacy, safety and costs of drugs for H. pylori infection lists preferred regimens, usually include metronidazole [500 mg twice daily for 10-14 days] or tinidazole at the same dosage, whose adverse effects can include metallic taste, reactions to alcohol, seizures and neuropathy).
- Pandey S, Gupta GK, Wanjari SJ, Nijhawan S. Comparative study of tinidazole versus metronidazole in treatment of amebic liver abscess: A randomized control trial. Indian J Gastroenterol. 2018;37:196–201. [PubMed: 29948994](Among 150 patients with amebic liver abscess treated with metronidazole [14 days] or tinidazole [5 days], time to response was better with tinidazole but ultimate response rates were similar, although metronidazole was associated with a higher rate of adverse events including nausea, vomiting and metallic taste resulting in early discontinuation in 10%; no mention of ALT elevations or hepatotoxicity).
- Shamberg L, Vaziri H. Hepatotoxicity of Inflammatory Bowel Disease Medications. J Clin Gastroenterol. 2018;52:674–84. [PubMed: 30036242](Review of the hepatotoxicity of drugs used for inflammatory bowel disease mentions that metronidazole has been reported to cause liver injury, but it is rare and does not justify prospective monitoring during therapy).
- Ataee P, Karimi A, Eftekhari K. Hepatic failure following metronidazole in children with Cockayne syndrome. Case Rep Pediatr. 2020;2020:9634196. [PMC free article: PMC7008298] [PubMed: 32082677](Two cases: 2 and 5 years old boys with type B [infant onset] Cockayne syndrome developed jaundice, vomiting and agitation 2 and 3 days after starting oral metronidazole for minor infection [bilirubin 13.7 and 16.0 mg/dL, ALT 2815 and 2248 U/L, Alk P 1265 and 1654 U/L, INR 5.1 and 4.6], one dying within 2 days of admission and the second recovering but with persistent jaundice and serum enzyme elevations one month later; whether similar reactions occur with tinidazole is uncertain).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Effects of Tinidazole on Food Intake in Chinchillas (Chinchilla lanigera).[J Am Assoc Lab Anim Sci. 2021]Effects of Tinidazole on Food Intake in Chinchillas (Chinchilla lanigera).Tournade CM, Fink DM, Williams SR, Mans C. J Am Assoc Lab Anim Sci. 2021 Sep 1; 60(5):587-591. Epub 2021 Jul 29.
- Review Secnidazole.[LiverTox: Clinical and Researc...]Review Secnidazole.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Benznidazole.[LiverTox: Clinical and Researc...]Review Benznidazole.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Tinidazole: from protozoa to Helicobacter pylori--the past, present and future of a nitroimidazole with peculiarities.[Expert Rev Anti Infect Ther. 2...]Review Tinidazole: from protozoa to Helicobacter pylori--the past, present and future of a nitroimidazole with peculiarities.Manes G, Balzano A. Expert Rev Anti Infect Ther. 2004 Oct; 2(5):695-705.
- Review Tinidazole: a nitroimidazole antiprotozoal agent.[Clin Ther. 2005]Review Tinidazole: a nitroimidazole antiprotozoal agent.Fung HB, Doan TL. Clin Ther. 2005 Dec; 27(12):1859-84.
- Tinidazole - LiverToxTinidazole - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...