NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Secnidazole is an orally available, broad spectrum antimicrobial agent used in the treatment of bacterial vaginosis. Secnidazole is a nitroimidazole similar to metronidazole but is used as a single dose and, unlike metronidazole, has not been linked to serum enzyme elevations during therapy or to cases of clinically apparent acute liver injury.
Background
Secnidazole (sek nid' a zole) is an oral, broad spectrum antimicrobial that has activity against bacteria as well as several parasites and is used for therapy of suspected bacterial vaginosis. Secnidazole is a nitroimidazole similar to metronidazole and is activated intracellularly by bacterial or parasitic enzymes to a radical anion, which damages large protein molecules and DNA. Secnidazole has been available in many other countries for decades but was first approved for use in the United States in 2017 for therapy of bacterial vaginosis. Secnidazole is available in 2 gram packets of granules under the brand name Solosec. The recommended dose is a one-time dose of 2 grams, the granules sprinkled on soft food and taken within 30 minutes. Secnidazole is generally well tolerated, but side effects can include metallic or bitter taste, headache, nausea, diarrhea, abdominal pain and vulvovaginal candidiasis.
Hepatotoxicity
Secnidazole is typically given as a single oral dose and, in follow up, de novo serum enzyme elevations occur rarely, overall rates being less than 0.5% of participants. In prelicensure studies, there were no reported cases of clinically apparent liver injury. Furthermore, despite considerable use worldwide, secnidazole has not been linked convincingly to instances of clinically apparent liver injury with jaundice.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The cause of rare instances of serum enzyme elevations with secnidazole therapy is unknown. Its lack of hepatotoxicity probably relates to being given in a single dose. Other nitroimidazoles such as ornidazole and metronidazole have been implicated in cases of acute liver injury with jaundice, but generally with more prolonged use. Secnidazole is metabolized in the liver but has not been found to affect cytochrome P450 enzymes and has no known significant drug-drug interactions.
Drug Class: Antiinfective Agents; Gastrointestinal Agents
Other Drugs in the Subclass, Nitroimidazoles: Benznidazole, Metronidazole, Tinidazole
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Secnidazole – Generic, Solosec®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
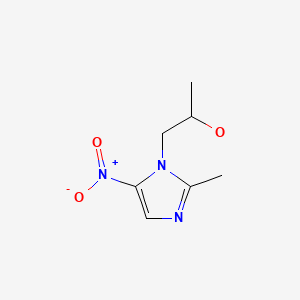
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Secnidazole | 3366-95-8 | C7-H11-N3-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 25 February 2020
- Zimmerman HJ. Hepatic injury from the treatment of infectious and parasitic diseases. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 589-637.(Expert review of hepatotoxicity of antibiotics published in 1999; does not mention secnidazole).
- Moseley RH. Antibacterial and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013, pp. 463-81.(Review of hepatotoxicity of antibacterial medications; does not discuss secnidazole).
- Wetzel DM, Phillips MA. Chemotherapy of protozoal infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 987-1000.(Textbook of pharmacology and therapeutics).
- Rosenthal PJ. Antiprotozoal drugs. In, Katzung BG, Masters SB, Trevor AJ. Basic and clinical pharmacology. 12th ed. New York: McGraw Hill, 2012, pp. 915-36.(Textbook of pharmacology and therapeutics).
- FDA. Drugs@FDA: FDA-Approved Drugs. Available at: https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that ALT elevations arose in 3% to 6% of patients receiving secnidazole, but in a similar proportion on placebo [5%] and there were no cases with accompanying jaundice and symptoms). - Roe FJ. Safety of nitroimidazoles. Scand J Infect Dis Suppl. 1985;46:72–81. [PubMed: 3865353](Review of the safety of secnidazole and other nitroimidazoles mentions side effects of nausea, headaches, metallic taste, furred tongue, itching and skin rash, but does not discuss ALT elevations or hepatotoxicity).
- Gillis JC, Wiseman LR. Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis. Drugs. 1996;51:621–38. [PubMed: 8706597](Review of the mechanism of action, pharmacology, clinical efficacy and tolerance of secnidazole; mentions that adverse events are generally mild and similar to those of other nitroimidazoles; no mention of ALT elevations or hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to a nitroimidazole antibiotic).
- Bohbot JM, Vicaut E, Fagnen D, Brauman M. Treatment of bacterial vaginosis: a multicenter, double-blind, double-dummy, randomised phase III study comparing secnidazole and metronidazole. Infect Dis Obstet Gynecol. 2010;2010:705692. pii. [PMC free article: PMC2946572] [PubMed: 20885970](Among 577 women with suspected bacterial vaginosis treated with a single oral dose of secnidazole or a 7-day course of oral metronidazole, day 28 success rates were similar in the two groups [60.1% vs 59.5%] and adverse event rates were similar; no mention of ALT elevations or hepatotoxicity).
- Pasupuleti V, Escobedo AA, Deshpande A, Thota P, Roman Y, Hernandez AV. Efficacy of 5-nitroimidazoles for the treatment of giardiasis: a systematic review of randomized controlled trials. PLoS Negl Trop Dis. 2014;8:e2733. [PMC free article: PMC3953020] [PubMed: 24625554](Systematic review of 30 randomized controlled trials in 3930 patients comparing nitroimidazoles including secnidazole to placebo in giardiasis; mentions that harmful outcomes were uncommon and side effects reported more commonly than with placebo included only metallic taste and headache; no mention of ALT levels or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to metronidazole or other nitroimidazoles).
- Hillier SL, Nyirjesy P, Waldbaum AS, Schwebke JR, Morgan FG, Adetoro NA, Braun CJ. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379–86. [PubMed: 28697102](Among 215 women with bacterial vaginosis enrolled in a randomized control trial, clinical cure was achieved in 49% receiving 1 g and 65% receiving 2 g of secnidazole vs 19% receiving placebo; while side effects were generally mild, there were no severe adverse events, and only 1 patient had elevations in serum ALT levels).
- Schwebke JR, Morgan FG Jr, Koltun W, Nyirjesy P. A phase-3, double-blind, placebo-controlled study of the effectiveness and safety of single oral doses of secnidazole 2 g for the treatment of women with bacterial vaginosis. Am J Obstet Gynecol. 2017;217:678.e1. [PubMed: 28867602](Among 189 women with bacterial vaginosis treated with a single 2 g dose of secnidazole or placebo, response rates were 53% vs 19% and adverse events were generally mild, and there were no serious adverse events and there were no “notable differences” in changes from baseline in “clinical laboratory parameters” between the two groups).
- Chavoustie SE, Gersten JK, Samuel MJ, Schwebke JR. A phase 3, multicenter, prospective, open-label study to evaluate the safety of a single dose of secnidazole 2 g for the treatment of women and postmenarchal adolescent girls with bacterial vaginosis. J Womens Health (Larchmt). 2018;27:492–7. [PubMed: 29323627](Among 283 women with bacterial vaginosis treated with a single dose of secnidazole oral granules, 72.5% had an adequate clinical response and adverse events were mild-to-moderate in severity; no mention of ALT elevations or hepatotoxicity).
- Secnidazole (Solosec) for bacterial vaginosis. Med Lett Drugs Ther. 2018;60(1543):52–3. [PubMed: 29635264](Concise review of the mechanism of action, clinical efficacy, safety and costs of secnidazole shortly after its approval in the US as therapy of bacterial vaginosis; mentions side effects of headache, nausea, diarrhea, abdominal pain, vulvovaginal pruritus and dysgeusia; no mention of ALT elevations or hepatotoxicity).
- Pentikis HS, Adetoro N. Two phase 1, open-label, single-dose, randomized, crossover studies to assess the pharmacokinetics, safety, and tolerability of orally administered granules of secnidazole (2 g) in healthy female volunteers under different administration conditions. Clin Pharmacol Drug Dev. 2018;7:543–53. [PMC free article: PMC6033001] [PubMed: 29125715](Two crossover pharmacokinetic studies of single doses of granules of secnidazole in 48 healthy controls reported adverse events including headache [46%], constipation [21%], somnolence [19%], nausea [15%] and abdominal pain [6%], and “no clinically significant changes in laboratory … findings were observed”).
- Elghazaly SM, Hamam KM, Badawy MM, Yakoub Agha NA, Samy A, Abbas AM. Efficacy and safety of single dose of oral secnidazole 2 g in treatment of bacterial vaginosis: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2019;238:125–31. [PubMed: 31129560](Systematic review of 6 trials of secnidazole, metronidazole and ornidazole for bacterial vaginosis found similar adverse event rates, but no details given and no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis.[Drugs. 1996]Review Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis.Gillis JC, Wiseman LR. Drugs. 1996 Apr; 51(4):621-38.
- Susceptibility of bacterial vaginosis (BV)-associated bacteria to secnidazole compared to metronidazole, tinidazole and clindamycin.[Anaerobe. 2017]Susceptibility of bacterial vaginosis (BV)-associated bacteria to secnidazole compared to metronidazole, tinidazole and clindamycin.Petrina MAB, Cosentino LA, Rabe LK, Hillier SL. Anaerobe. 2017 Oct; 47:115-119. Epub 2017 May 15.
- A phase-3, double-blind, placebo-controlled study of the effectiveness and safety of single oral doses of secnidazole 2 g for the treatment of women with bacterial vaginosis.[Am J Obstet Gynecol. 2017]A phase-3, double-blind, placebo-controlled study of the effectiveness and safety of single oral doses of secnidazole 2 g for the treatment of women with bacterial vaginosis.Schwebke JR, Morgan FG Jr, Koltun W, Nyirjesy P. Am J Obstet Gynecol. 2017 Dec; 217(6):678.e1-678.e9. Epub 2017 Sep 1.
- Secnidazole: next-generation antimicrobial agent for bacterial vaginosis treatment.[Future Microbiol. 2018]Secnidazole: next-generation antimicrobial agent for bacterial vaginosis treatment.Nyirjesy P, Schwebke JR. Future Microbiol. 2018 Apr; 13:507-524. Epub 2018 Jan 12.
- Review Benznidazole.[LiverTox: Clinical and Researc...]Review Benznidazole.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Secnidazole - LiverToxSecnidazole - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...